Surgical treatment of Loeys-Dietz syndrome in a 3-year-old: case report and review of literature
Introduction
Loeys-Dietz syndrome (LDS) is an autosomal dominant disorder of the connective tissue that was described in 2005 (1). Patients with mutations in the TGFBR1, TGFBR2, TGFB2, TGFB3, and SMAD3 genes were noted to present with phenotypical features of LDS. LDS affects tissues and organs throughout the body, especially the great arteries. Rapidly progressive aortic aneurysmal disease is a distinct feature of LDS and thus mandates close monitoring of these patients. Early prophylactic surgery is crucial to avoid catastrophic aortic complications and achieve a good long-term prognosis (2). Here we present a case of a young child with LDS who was implanted a composite valve-graft by a St Jude Regent 21# mechanical valve seated within a 24 mm Gore-Tex graft to finish the Bentall procedure and achieved a favourable post-operative follow-up outcome, describe the presenting signs and symptoms, and discuss the clinical management. We present the following case in accordance with the CARE reporting checklist (3) (available at http://dx.doi.org/10.21037/tp-20-146).
Case presentation
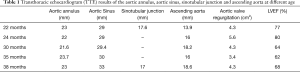
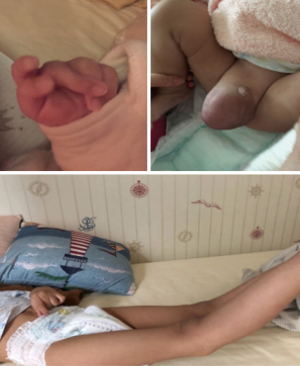
Written informed consent was obtained from the patient's parents. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Guangdong Provincial People’s Hospital ethical review committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this Case report and any accompanying images. A three-and-half year-old-boy with a history of LDS was referred for surgical intervention after a transthoracic echocardiogram (TTE) demonstrated a dilated aortic annulus (diameter: 23 mm, z score 11.59) and ascending aorta (diameter:18.6 mm, z score 3.99) as well as severe regurgitation and left ventricle dilation and decreasing systolic function (Table 1). Notably, on physical examination, he was noted to have middle finger spasm bilaterally, inguinal hernia, and hyperextensibility of the knees (Figure 1). At 7 months of age, an echocardiogram demonstrated left ventricular enlargement, a patent foramen ovale, aortic sinus dilatation (diameter: 24 mm), ascending aortic dilation (diameter: 14 mm), and moderate regurgitation of aortic valve (regurgitation area: 1.5 cm2). Based on these echocardiogram findings, gene sequencing was performed at that time and revealed a heterozygous missense mutation of the TGFBR2 gene: 1634G-->T, leading to an amino acid change of C545F (Table 2). Parental gene sequencing did not reveal any abnormalities. Given the physical signs, echocardiogram findings, and genomic studies, a diagnosis of LDS type 2 (OMIM: 610168) was made.
Full table

Full table
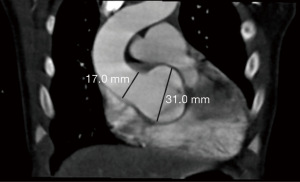
The child’s aortic regurgitation was monitored closely, and serial TTE over the first three years of life demonstrated progressive dilation of the aortic annulus, aortic sinus, sinotubular junction, and ascending aorta (Table 1). Further, aortic regurgitation progressively worsened (Table 1). A CT of the head and neck demonstrated an enlarged skull and no obvious dilation or deformity of the head and neck blood vessels. A CT at 2 years of age demonstrated a dilated aortic annulus and ascending aorta (Figure 2).
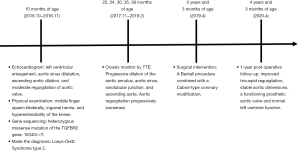
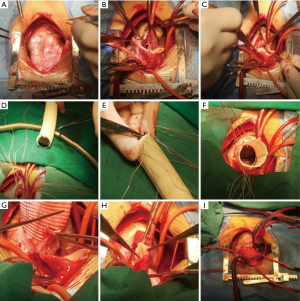
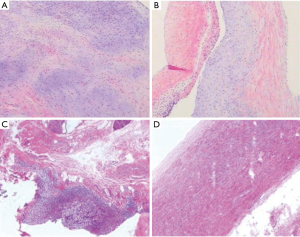
Because of the severe aortic valve regurgitation resulting in rapid and progressive left ventricle dilation, the patient underwent a Bentall procedure at 3 years and 3 months of age. His extremely dilated aortic annulus was felt to be a contraindication to a valve sparing aortic root replacement procedure. After coronary buttons were harvested and diseased sinus tissue resected (Figure 3A,B,C), a composite valve-graft by a St Jude Regent 21# mechanical valve seated within a 24 mm Gore-Tex graft was implanted (Figure 3D,E,F). Given the distance between the coronary buttons and the aortic graft, Cabrol extension of the coronary arteries was performed (Figure 3G,H,I). Postoperative transesophageal and transthoracic echocardiography demonstrated good aortic valve function with no perivalvular regurgitation and laminar coronary flow. The cardiopulmonary bypass time was 193 min and the aortic cross clamp time was 121 min. Pathological examination revealed that the aortic valve had undergone degeneration with interstitial fibrous hyperplasia, focal collagenization, myxoid degeneration and focal calcification (Figure 4A). Furthermore, the resected aneurysmal aorta revealed a thin wall with inflammatory infiltrate (Figure 4B). The patient had an uneventful postoperative recovery and was discharged on postoperative day 12 with losartan and warfarin. The timeline of this case was shown in Figure 5.
At 3 months follow-up, TTE demonstrated normal left ventricle function, a functioning prosthetic aortic valve and mild tricuspid regurgitation. The proximal part of the ascending aorta diameter was 21.2 mm and a mild gradient was measured in the proximal aortic arch (Vmax 1.6 m/s). At 1-year post repair, the patient continued to do well with improved tricuspid regurgitation, stable aortic dimensions, a functioning prosthetic aortic valve and normal left ventricle function.
Discussion
LDS was originally described in 16 individuals from ten families (4). Patients with LDS exhibit a variety of features, mainly involving the cardiovascular, musculoskeletal and central nervous systems (5). TGFBR1 and TGFBR2 mutations were initially shown to cause LDS, with subsequent pathogenic mutations identified in SMAD3, TGFB2, and TGFB3. Pathogenic mutations in all of these genes, which encode mediators of the TGF signaling pathway, result in loss of function of the protein product (6), causing elastin disarray, loss of elastic fiber architecture and increased collagen expression in the aortic media of patients. The aortic wall is characterized by medial diffuse degeneration and elastin fragmentation (7). Because of these structural changes, the vascular media weakens and makes the aorta prone to aneurysmal dilation and dissection (4,5,8).
Half of LDS patients with severe craniofacial features are at particularly high risk, known to have aneurysm rupture at an early age and at smaller dimensions than those with other aneurysm syndromes (1,4). It has been reported that patients with LDS undergo cardiovascular operations earlier (mean age, 16.9 years) and die earlier (mean age, 22.6 years) but have a lower intraoperative mortality rate (9). The reported median age at first dissection for patients with LDS is 26.7 years (10) and the mean age of death was 26 years and the median survival was 37 years. The leading cause of death for LSD was dissection of the thoracic aorta (67%), abdominal aorta (22%), and the cerebral vasculature (7%) (4). The vascular phenotype of LDS, characterized by aneurysmal changes and tortuosity of the arteries, is not limited to the aortic root, and lifelong serial imaging of the entire arterial tree, both systemic and pulmonary, is mandatory (11).
The decision to undergo aortic surgery is typically based on the absolute dimension of the aorta, rate of progression, aortic valve function, severity of noncardiac features, family history, and information about genotype (4,12). A clinical study including 53 LDS patients, 33 of whom underwent aortic surgery noted that close monitoring and early prophylactic interventions were the key to avoid aortic catastrophe and to achieve a good long-term prognosis (2).
This child is one of the youngest LDS patients to receive a Bentall procedure. Progressive aortic dilation and severe aortic valve insufficiency mandated early intervention. Fortunately for this patient, we were able to implant an adult-sized valved conduit. However, the consequence of this operation is that this child will need life-long anticoagulation.
Early genetic testing is warranted if the physical examination and history are suggestive of LDS. Further, genetic testing will permit the differentiation of these patients from those with Marfan syndrome. Close follow up is required for children with LDS, provided there is no life-threatening dissection or aneurysm in the aorta and the function of the aortic valve is not significantly impaired.
Children with LDS and aortic dilatation and aortic valve regurgitation should be closely followed. Treatment with a Bentall procedure or valve sparing root replacement are required and adult-sized grafts and valves will minimize the need for reintervention as the child grows. Continued life-long follow-up in these patients is needed to monitor the rest of the arterial tree, which is susceptible to aneurysmal dilation and complications and may require prophylactic intervention.
The limitation of this study is that this patient should need life-long anticoagulation and we can’t predict whether and when other arteries need intervention. We need the long-term follow-up consequence to evaluate our surgical strategies.
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China (2018YFC1002600) and the Science and Technology Planning Project of Guangdong Province, China (2017A070701013, 2017B090904034, 2017B030314109, 2019B020230003) and the Guangdong peak project (DFJH201802)
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-146
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-146). MSS serves as an unpaid Deputy Editors-in-Chief of Translational Pediatrics from Dec 2018 to Nov 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Guangdong Provincial People’s Hospital ethical review committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this Case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275-81. [Crossref] [PubMed]
- Aftab M, Cikach FS, Zhu Y, et al. Loeys-Dietz syndrome: Intermediate-term outcomes of medically and surgically managed patients. J Thorac Cardiovasc Surg 2019;157:439-50.e5. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788-98. [Crossref] [PubMed]
- Choo JT, Tan TH, Lai AH, et al. Loeys-Dietz syndrome: a Marfan-like syndrome associated with aggressive vasculopathy. Singapore Med J 2009;50:e353-7. [PubMed]
- Zimmermann MT, Urrutia RA, Blackburn PR, et al. Novel Pathogenic Variant in TGFBR2 Confirmed by Molecular Modeling Is a Rare Cause of Loeys-Dietz Syndrome. Case Rep Genet 2017;2017:7263780. [Crossref] [PubMed]
- Iba Y, Minatoya K, Matsuda H, et al. Surgical experience with aggressive aortic pathologic process in Loeys-Dietz syndrome. Ann Thorac Surg 2012;94:1413-7. [Crossref] [PubMed]
- Sim HT, Seo DJ, Yu JJ, et al. Valve Sparing Aortic Root Replacement in Children with Loeys-Dietz Syndrome. Korean J Thorac Cardiovasc Surg 2015;48:272-6. [Crossref] [PubMed]
- Nakajima T, Tachibana K, Miyaki Y, et al. Acute dilatation of the ascending aorta and aortic valve regurgitation in Loeys-Dietz syndrome. Ann Thorac Surg 2014;97:2188-90. [Crossref] [PubMed]
- Inoue Y, Minatoya K, Oda T, et al. Total Aortic Replacement for a 9-Year-Old Boy With Loeys-Dietz Syndrome. Ann Thorac Surg 2016;101:1185-8. [Crossref] [PubMed]
- Rizzo S, Stellin G, Milanesi O, et al. Aortic and Pulmonary Root Aneurysms in a Child With Loeys-Dietz Syndrome. Ann Thorac Surg 2016;101:1193-5. [Crossref] [PubMed]
- Arslan-Kirchner M, Epplen JT, Faivre L, et al. Clinical utility gene card for: Loeys-Dietz syndrome (TGFBR1/2) and related phenotypes. Eur J Hum Genet 2011;19. [Crossref] [PubMed]