Preface to the column “Metabolism of Childhood Cancer”
Reprogramming of cellular energy metabolism is a central theme of cancer cells, as described by Warburg (1) (Figure 1) and designated as a hallmark of cancer by Hanahan and Weinberg (2). Indeed, cancer has been called a metabolic disease (3-8). Given the significance of metabolic alterations in cancer, this special column on “Metabolism of Childhood Cancer” is launched and I am delighted to serve as the column editor. The column initiates with three review articles authored by Nguyen and Zhu from University of Texas Southwestern Medical Center; Tech and Gershon from University of North Carolina School of Medicine; and Aminzadeh, Vidali et al. from Paracelsus Medical University being presented in this issue of Translational Pediatrics.
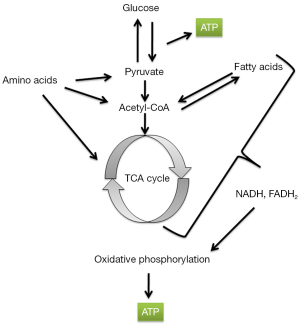
Specific metabolic aberrations are now known to be associated with adult cancers. For example, increased abundance of 2-hydroxyglutarate (2HG), caused either by gain-of-function mutations in the 2HG-producing enzymes isocitrate dehydrogenase 1 (IDH1) and IDH2, or reduced expression of or loss-of-function mutations in enzymes such as L-2-hydroxyglutarate dehydrogenase (L-2HGDH) that degrade this oncometabolite, are associated with brain tumors, acute myeloid leukemias, intrahepatic cholangiocarcinomas, central chondrosarcomas, and clear cell renal cell carcinomas (9-16). Mutations in nuclear genes encoding subunits of succinate dehydrogenase (SDH) have been found in pheochromocytomas and paragangliomas (17-19). Fumarate hydratase (FH) mutations are associated with a familial syndrome of uterine fibroids, skin leiomyomata, and adult-type renal papillary carcinoma (20). These mutations in metabolic enzymes and/or increased levels of oncometabolites influence the development of cancer, often through effecting epigenetic changes in the cancer cell (15,21-24). Indeed, these metabolic changes are being recognized as characteristics of specific cancers that produce diagnostically and prognostically useful biomarkers (25-28) and as vulnerabilities in the affected cancer cell that can be exploited for therapeutic purposes (29,30).
The importance of studying metabolism in specific cancers that affect children is now being recognized in earnest, as is evident from the first three articles of the column.
Nguyen and Zhu review the current understanding of the Lin28/let-7 axis and its role in tumorigenesis in general and in pediatric liver cancer in particular. For example, they discuss how the Lin28/let-7 axis may promote cancer growth by reprograming metabolism through upregulation of pyruvate dehydrogenase kinase 1 (PDK1), which in turn inactivates pyruvate dehydrogenase (PDH) thereby restricting the conversion of pyruvate to acetyl-CoA, which then is not available to enter the Krebs cycle. Zhu has a long-standing interest in this field and with his colleagues has previously shown that Lin28/let-7 axis regulates glucose metabolism and has a role to play not only in liver cancer but also in Wilms tumor and germ cell tumors (31-35).
Aminzadeh, Vidali, Sperl, Kofler, and Feichtinger review the features of energy metabolism in two common childhood solid tumors—neuroblastoma and Wilms tumor. Both tumors show alterations in oxidative phosphorylation and glycolysis that, as the authors discuss, are linked to the underlying molecular alterations, such as amplification of MYCN in high-risk neuroblastomas and the activation of the WNT/β-catenin pathway in Wilms tumors.
Tech and Gershon review the alterations of energy metabolism in medulloblastoma, another common solid tumor of childhood. They discuss how the metabolic features of medulloblastoma recapitulate the metabolic features of neural progenitors, focusing particularly on Sonic hedgehog (Shh) signaling associated induction of lipogenesis and aerobic glycolysis. Indeed, this model of similarity between fetal development and cancer may apply to other cancers as well, because the same signaling pathways that drive fetal growth are reactivated in cancers.
I would like to thank all the authors for their superb contributions and to Nancy Q. Zhong and the members of the Translational Pediatrics editorial team for their diligent work in bringing this important topic to the readers of this journal.
References
- Warburg O. On the origin of cancer cells. Science 1956;123:309-14. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Seyfried TN, Flores RE, Poff AM, et al. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014;35:515-27. [PubMed]
- Coller HA. Is cancer a metabolic disease? Am J Pathol 2014;184:4-17. [PubMed]
- Hainaut P, Plymoth A. Cancer as a metabolic disease. Curr Opin Oncol 2012;24:56-7. [PubMed]
- Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) 2010;7:7. [PubMed]
- Booyens J, Katzeff IE. Cancer: a simple metabolic disease? Med Hypotheses 1983;12:195-201. [PubMed]
- Kuhlmey W. Cancer as metabolic disease. Krebsarzt 1958;13:331-7. [PubMed]
- Aghili M, Zahedi F, Rafiee E. Hydroxyglutaric aciduria and malignant brain tumor: a case report and literature review. J Neurooncol 2009;91:233-6. [PubMed]
- Frezza C, Tennant DA, Gottlieb E. IDH1 mutations in gliomas: when an enzyme loses its grip. Cancer Cell 2010;17:7-9. [PubMed]
- Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 2010;207:339-44. [PubMed]
- Rakheja D, Konoplev S, Su M, et al. High incidence of IDH mutations in acute myeloid leukaemia with cuplike nuclei. Br J Haematol 2011;155:125-8. [PubMed]
- Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012;17:72-9. [PubMed]
- Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 2011;224:334-43. [PubMed]
- Shim EH, Livi CB, Rakheja D, et al. L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov 2014;4:1290-8. [PubMed]
- Rakheja D, Medeiros LJ, Bevan S, et al. The emerging role of d-2-hydroxyglutarate as an oncometabolite in hematolymphoid and central nervous system neoplasms. Front Oncol 2013;3:169. [PubMed]
- Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000;287:848-51. [PubMed]
- Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 2000;26:268-70. [PubMed]
- Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001;69:49-54. [PubMed]
- Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002;30:406-10. [PubMed]
- Waterfall JJ, Killian JK, Meltzer PS. The role of mutation of metabolism-related genes in genomic hypermethylation. Biochem Biophys Res Commun 2014;455:16-23. [PubMed]
- Yang M, Soga T, Pollard PJ, et al. The emerging role of fumarate as an oncometabolite. Front Oncol 2012;2:85. [PubMed]
- Kaelin WG Jr. Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol 2011;76:335-45. [PubMed]
- Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011;19:17-30. [PubMed]
- Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012;18:624-9. [PubMed]
- Borger DR, Goyal L, Yau T, et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin Cancer Res 2014;20:1884-90. [PubMed]
- Janin M, Mylonas E, Saada V, et al. Serum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association group. J Clin Oncol 2014;32:297-305. [PubMed]
- DiNardo CD, Propert KJ, Loren AW, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood 2013;121:4917-24. [PubMed]
- Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013;340:626-30. [PubMed]
- Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science 2013;340:622-6. [PubMed]
- Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes Dev 2014;28:971-82. [PubMed]
- Shinoda G, Shyh-Chang N, Soysa TY, et al. Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells 2013;31:1563-73. [PubMed]
- Zhu H, Shyh-Chang N, Segrè AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011;147:81-94. [PubMed]
- West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 2009;460:909-13. [PubMed]
- Nguyen LH, Robinton DA, Seligson MT, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell 2014;26:248-61. [PubMed]

Departments of Pathology and Pediatrics, University of Texas Southwestern Medical Center, Dallas, Texas, 75390, USA; Department of Pathology and Laboratory Medicine, Children’s Health, Dallas, Texas, 75235, USA. (Email: dinesh.rakheja@utsouthwestern.edu.)


