
Association between vaccinations and clinical manifestations in children with COVID-19
Introduction
A novel coronavirus outbreak occurred in December 2019 (1-4). As of May 30, 2020, a total of 5,817,385 Coronavirus Disease 2019 (COVID-19) cases and 362,705 related deaths have been confirmed (5). Common symptoms at onset of illness include fever, cough, myalgia, and fatigue (6-8), whereas less common symptoms include shortness of breath, dizziness, headache, pharyngalgia, chest pain, abdominal pain, diarrhea, nausea, vomiting, loss of appetite, and weakness (1,2). COVID-19 is more likely to affect older patients with comorbidities (9), as only 889 of 72,314 (1.2%) such adult cases were asymptomatic cases (10,11). In contrast, 12.9% of pediatric cases have been asymptomatic cases (12).
It has remained unclear as to why the infection rate of COVID-19 in children has been less than that in adults. One possible reason is that children have less exposure and more protection by their guardians; another possibility is that children have more active innate immune responses (13). Most infants have received regular immunizations in China and other Asian countries, including Bacillus Calmette Guerin (BCG), which has been demonstrated to provide non–specific protection against influenza infections, possibly via the induction of trained innate immunity (14). However, the underlying features of such BCG-mediated protection and the association of BCG vaccinations and clinical manifestations in children with COVID-19 have remained largely unknown (15,16). Therefore, in the present study, we explored the associations of BCG vaccination and clinical manifestations in pediatric patients with COVID-19.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-225).
Methods
Patient and data selection
All pediatric patients with COVID-19 were recruited from two hospitals during the specific period from January 28 to March 12, including 240 laboratory—confirmed cases from Wuhan Children’s Hospital, 56 suspected cases and 8 imported confirmed cases from Shanghai Children’s Medical Center. Wuhan Children’s Hospital represents the only hospital in Wuhan for treating pediatric patients under 16 years with COVID-19, as designated by the Chinese central government. We collected data on demographics (age, gender), epidemiological histories, clinical symptoms, results of clinical pathogen examinations from hospital information system or laboratory information system. The vaccination status (influenza vaccines and Bacillus Calmette Guerin vaccines) was checked in the vaccination management system and information of unavailable patients in the system was collected from the parents by telephone, however the type and vaccinating time of influenza virus were not collected. The total vaccination times for BCG were calculated from the time of initial vaccination time to the time of confirmation of diagnosis.
Diagnostic criteria
Suspected and confirmed cases were diagnosed based on the Novel Coronavirus 2019 Diagnosis and Treatment Protocol, seventh version (17). Nasopharyngeal swabs from suspected children younger than two years, as well as throat swabs from children two years or older, were obtained for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). According to the protocol of the Chinese Center for Disease Control and Prevention (CDC) for detection of SARS-CoV-2, a duplex one-step real-time reverse-transcription PCR (RT-PCR) was performed to confirm SARS-CoV-2 infection at the local designated laboratory, for which positive detection determined a confirmed case. Suspected cases were determined based on one of the following two criteria: (I) having an epidemiological link to adult cases; or (II) an exposure to Wuhan or other epidemic areas in the Hubei province within the previous 14 days and presenting with acute fever and/or respiratory symptoms (18). Further pathogens tests were needed for other respiratory viruses in the following, asymptomatic case is positive for SARS-CoV-2 infection and not any symptoms at the initial admission to the outpatient, serologic tests in the suspected cases were not conducted since it was not available. We compared the infection rate between patients with and without vaccinated BCG or flu vaccine.
Statistical analysis
We first described the demographic characteristics of patients, including gender and age. Subsequently, we focused on the association of initial symptoms with age, vaccinations. Chi-square tests and Fisher’s exact tests were used for categorical variables when appropriate, and Mann-Whitney U tests were used for comparing median values of non-normally distributed variables. All analyses were conducted using Statistical Product and Service Solutions (SPSS 25.0) software and R 3.6.2. The criterion for statistical significance was P<0.05 via two-tailed tests.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board of Wuhan Children’s Hospital (IEC-2020R003-E01) and Shanghai Children’s Medical Center (SCMCIRB-K2020019-1). Individual consent for this retrospective analysis was waived.
Results
A total of 304 pediatric patients with COVID-19 were included in this study (Table 1), including 56 suspected and 248 laboratory-confirmed cases. Among these cases, 240 (97.17%) children with COVID-19 were recruited from Wuhan Children’s Hospital, and there were also eight imported cases (2.83%, Table 2). All suspected cases were collected from the Shanghai Children’s Medical Center. Among them, 153 (61.69%) confirmed and 33 suspected (58.93%) male patients were included, which were more than those of female cases. The median age (interquartile range) was 7.18 (2.24–10.89) years for confirmed cases and 4.92 (1.82–7.48) years for suspected children. Furthermore, 92 of 304 (30.26%) cases consisted of asymptomatic patients. The percentages of initial symptoms were significantly different (P<0.001) between confirmed and suspected cases. Total 181/240 (75.42%) vaccination status was founded in the vaccination management system, that of 59/240 (24.58%), 8 imported confirmed cases and all 56 suspected cases was collected from the guardians by telephone.
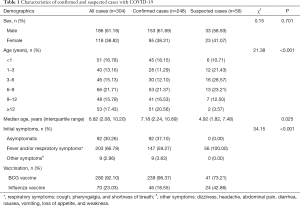
Full table
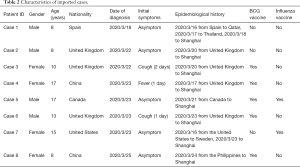
Full table
The percentage of asymptomatic children increased with age; in contrast, the percentage of symptomatic patients with COVID-19 decreased with age among both boys and girls (P<0.001) (Figure 1). The median age (interquartile range) was 9.28 (6.31–12.54) years for asymptomatic confirmed children and 4.57 (0.95–9.33) years for symptomatic children (P<0.001), which was not significantly different from suspected cases.
The percentage of asymptomatic patients vaccinated with BCG was not significantly lower than the percentage of those without BCG vaccination [86/280 (30.71%) vs. 6/13 (46.15%), P=0.203]. Similarly, the percentage of patients with fever and/or respiratory symptoms who had been immunized with BCG was also not significantly higher than the percentage of those without BCG vaccination [187/280 (66.79%) vs. 5/13 (38.46%), P=0.033]. There was no significant difference in the percentage of asymptomatic patients given influenza vaccine compared to the percentage of those who did not receive an influenza vaccination (P=0.267) (Table 3).
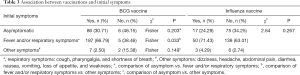
Full table
Using correlational analysis, we found that hs-CRP was correlated with the symptom of fever (r=0.31, P<0.001). Different ages were significantly associated with fever (P=0.004), cough (P=0.003), and diarrhea (P=0.012) in confirmed cases (Table 4). We have compared the eight imported cases with Chinese cases in Wuhan, the percentage of BCG vaccination (5/8) for imported confirmed cases is much lower than that of China (239/248), and the asymptomatic rate (5/8) is higher than that of Chinese cases (92/248). Meanwhile, age was found to be significantly associated with mycoplasma (P<0.001) and cytomegalovirus (P=0.040) infection in confirmed cases, co-infection was associated with hs-CRP between COVID-19 alone and in combination with MP (Table S1).
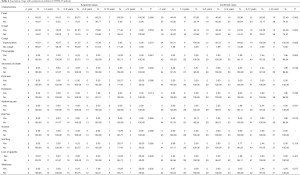
Full table
Discussion
To the best of our knowledge, this is the first study on the association of vaccinations and clinical manifestations in children infected with COVID-19. A previous study of 2,143 pediatric patients indicated that clinical manifestations in children infected with COVID-19 were less severe than those in adult patients (10,12). However, the underlying features of this phenomenon have not been well identified. In the present study, we found that pediatric patients with BCG vaccinations exhibited clinical features similar to those of patients who did not receive BCG vaccinations. Furthermore, the percentage of asymptomatic patients was positively correlated with age, suggesting that the severity of pediatric patients was related to the maturity of immune function.
In the current study, asymptomatic cases accounted for 37.10% of all confirmed cases, which is higher than the 12.90% reported among 2,143 pediatric patients in a previous study, since some clinical cases were also included in this previous study (12). The symptoms that children are more likely to be fever and/or cough than adults (19,20). We found that slightly more boys than girls (61.69% vs. 38.31%) were affected by COVID-19 and that the median age of all pediatric COVID-19 cases was 6.82 years (interquartile range: 2.08–10.20), which is similar to the findings of two recent epidemiological studies (4,12). Hence, these findings suggest that boys may be more exposed to family members and/or other children with COVID-19.
Although it remains unclear as to why symptoms in pediatric cases are milder than those in adult cases of COVID-19, this phenomenon may be related to both host and exposure factors. ACE2 or TMPRSS2 DNA polymorphisms were likely associated with genetic susceptibility of COVID-19 (21,22). The immune system of children is still developing and may respond to pathogens differently compared to that in adults. In the present study, we found that the percentage of asymptomatic COVID-19 infections increased with age, whereas the percentage of symptomatic COVID-19 infections decreased with age (Figure 1). It suggests that immune function gradually matures with age, and the more vigorous immune response mounted by adults may also explain the detrimental immune response associated with acute respiratory distress syndrome (13). Furthermore, since children often experience respiratory infections in the winter, we found that positive results of mycoplasma IgM were related to age in children with COVID-19. Children are also usually well cared for at home and might have relatively fewer opportunities to expose themselves to pathogens and/or sick patients, and girls are generally less exposed to outdoor activities than boys.
BCG has been identified to induce trained immunity that protects against unrelated pathogens (23,24). An analysis of infant immunization with BCG in 33 countries suggested BCG vaccination may reduce the incidence of acute lower respiratory infection by 17–37% (25). Children have a more active innate immune response and fewer underlying disorders. In the present study, we observed that the percentage of asymptomatic patients vaccinated with BCG was not significantly lower than those without BCG vaccination. This finding may be related to the trained immunity of BCG and any cross-protective non-specific effects: the immune system learns more than specific prevention from an intervention. Such training may enhance or reduce susceptibility to unrelated infections (26).
The present study has several strengths. First, this is the first study on the association of vaccinations with symptoms in children with COVID-19. Our findings demonstrate that, compared with symptoms in pediatric cases without BCG vaccination, the severity of symptoms in COVID-19 pediatric cases with BCG vaccination was similar. Second, we detected common pathogens of respiratory diseases, such that we were able to analyze the relationship between coinfection status and the severity of COVID-19 cases. Finally, we included both confirmed and suspected COVID-19 cases from two different centers, which may help to reveal a comprehensive picture of pediatric patients with COVID-19.
This study also has a number of limitations. First, the number of children without BCG vaccination was limited because it is free and mandatory to vaccine BCG at birth in China according to the Chinese policy (27), the comparison with children in other countries should be increased and the results will be more credible, we will try our best to look up the potential cooperation pediatric hospital in future. Thirteen children were not vaccinated in the present study. Three cases in the present study were imported from Spain, the United Kingdom, and the United States of America. As an important issue, clinical features of COVID-19 in such children require further analysis in future studies. Finally, this was a retrospective study from two hospitals, and the epidemic of COVID-19 is ongoing, however in the current retrospective study, the symptoms and age were recorded in the electronic health record, and the vaccination status was recorded in the specific vaccine management system, the deviation is relatively low. To gain a better understanding of COVID-19 in children, more detailed information on patient vaccinations and clinical outcomes should be collected in future studies.
Children with COVID-19 play an important role in family clusters and in community transmission, especially within kindergartens, as well as primary and middle schools (28). Since vaccination plans are different between Asian and Western countries, more comparative studies on protection via vaccinations (e.g., BCG) in COVID-19 patients are needed in future studies, it may be helpful to control the COVID-19 epidemic in the global situation.
Conclusions
In conclusion, pediatric COVID-19 patients with BCG vaccinations exhibit clinical manifestations similar to those of patients who had not been vaccinated for BCG, and the severity of symptoms in pediatric patients may be related to the maturity of immune function.
Acknowledgments
We are grateful for the participation provided by the children included in this study, as well as their guardians. Additionally, we thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. There are 169 overlap cases between our study and another publication (Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020. doi: 10.1056/NEJMc2005073.) of our hospital. The focused question, research contents of these two studies were completely different.
Funding: This study was supported by National Science Foundation of China (81872637), Shanghai Municipal Commission of Health and Family Planning (201840324), Medical and Engineering Cooperation Project of Shanghai Jiao Tong University (YG2017ZD15), the Project of Shanghai Children’s Health Service Capacity Construction (GDEK201708), Science and Technology Development Program of Pudong Shanghai New District (PKJ2017-Y01), and Shanghai Professional and Technical Services Platform (18DZ2294100), Program of Shanghai Science and Technology Committee (19441904400), the Foundation of National Facility for Translational Medicine, Shanghai(TMSK-2020-124), the Key Subject Program for Clinical Nutrition from Shanghai Municipal Health Commission (2019ZB0103).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-225
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-225
Peer Review File: Available at http://dx.doi.org/10.21037/tp-20-225
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-225). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board of Wuhan Children’s Hospital (IEC-2020R003-E01) and Shanghai Children’s Medical Center (SCMCIRB-K2020019-1). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Chang D, Lin M, Wei L, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA 2020;323:1092-3. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Coronavirus disease 2019 (COVID-19) Situation Report – 131. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200530-covid-19-sitrep-131.pdf?sfvrsn=d31ba4b3_2
- Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020;382:1663-5. [Crossref] [PubMed]
- Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689-96. [Crossref] [PubMed]
- Castagnoli R, Votto M, Licari A, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr 2020;174:882-9. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)-China, 2020. China CDC Weekly 2020;2:113-22. [Crossref]
- Kelvin AA, Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis 2020;20:633-4. [Crossref] [PubMed]
- Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020;145:e20200702. [Crossref] [PubMed]
- Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19?. J Microbiol Immunol Infect 2020;53:371-2. [Crossref] [PubMed]
- Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and Adaptive Immune Memory: an Evolutionary Continuum in the Host's Response to Pathogens. Cell Host Microbe 2019;25:13-26. [Crossref] [PubMed]
- Weng CH, Saal A, Butt WW, et al. Bacillus Calmette-Guérin vaccination and clinical characteristics and outcomes of COVID-19 in Rhode Island, United States: a cohort study. Epidemiol Infect 2020;148:e140. [Crossref] [PubMed]
- Ten Doesschate T, Moorlag SJCFM, van der Vaart TW, et al. Two Randomized Controlled Trials of Bacillus Calmette-Guérin Vaccination to reduce absenteeism among health care workers and hospital admission by elderly persons during the COVID-19 pandemic: A structured summary of the study protocols for two randomised controlled trials. Trials 2020;21:481. [Crossref] [PubMed]
- Novel Coronavirus 2019 Diagnosis and Treatment Protocol, 7th version. 2020. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml
- Jiehao C, Jin X, Daojiong L, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 2020;71:1547-51. [Crossref] [PubMed]
- Wu Q, Xing Y, Shi L, et al. Coinfection and Other Clinical Characteristics of COVID-19 in Children. Pediatrics 2020;146:e20200961. [Crossref] [PubMed]
- Ma N, Li P, Wang X, et al. Ocular Manifestations and Clinical Characteristics of Children With Laboratory-Confirmed COVID-19 in Wuhan, China. JAMA Ophthalmol 2020;138:1079-86. [Crossref] [PubMed]
- Hou Y, Zhao J, Martin W, et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med 2020;18:216. [Crossref] [PubMed]
- Godri Pollitt KJ, Peccia J, Ko AI, et al. COVID-19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Hum Genomics 2020;14:17. [Crossref] [PubMed]
- Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science 2016;352:aaf1098. [Crossref] [PubMed]
- Benn CS, Netea MG, Selin LK, et al. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013;34:431-9. [Crossref] [PubMed]
- Hollm-Delgado MG, Stuart EA, Black RE. Acute lower respiratory infection among Bacille Calmette-Guérin (BCG)-vaccinated children. Pediatrics 2014;133:e73-81. [Crossref] [PubMed]
- Sankoh O, Welaga P, Debpuur C, et al. The non-specific effects of vaccines and other childhood interventions: the contribution of INDEPTH Health and Demographic Surveillance Systems. Int J Epidemiol 2014;43:645-53. [Crossref] [PubMed]
- Chinese Center of Diseases Control and Prevention. Standardized Operation Procedure of Vaccination. 2018. Available online: http://www.chinacdc.cn/jkzt/ymyjz/
- Wang G, Zhang Y, Zhao J, et al. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet 2020;395:945-7. [Crossref] [PubMed]



