Effectiveness and safety of methylphenidate and atomoxetine for attention deficit hyperactivity disorder: a systematic review
Attention deficit hyperactivity disorder (ADHD) is a common psychiatric disorder, affecting about 3% of adults and 5% of children and adolescents (1,2). ADHD is associated with a broad range of negative outcomes for affected subjects and puts a serious burden on families and the society. As a result, early identification and treatment of symptoms of ADHD is essential to effective management of this disorder.
ADHD implicates a challenge for social and academic development of affected children and leads to more life events in adulthood and developmental comorbidities (3,4). Spencer et al. (5) found that about 65% of the patients had one or more of concomitant diseases in addition to ADHD, which hampered the treatment efficacy in clinical settings. Effective treatment often includes pharmacotherapy with agents influencing neurotransmission (6). In recent years, concerns have been raised about available drugs for the treatment of ADHD including methylphenidate immediate-release tablets (IR-MPH), methylphenidate controlled-release tablets (OROS-MPH) and atomoxetine (atomoxetine hydrochloride capsules, AHC). MPH is recommended as the first-choice drug, while AHC is preferred in case of MPH-related side effects or the presence of comorbid tics, anxiety or substance abuse (7).
For school-aged children, stimulant agents are well-established as first-line pharmacotherapy. IR-MPH is the most commonly prescribed and best-studied stimulant medication and has been proved effective for treating ADHD (8). And OROS-MPH is a once-daily controlled-release formulation developed to overcome some of the limitations associated with IR-MPH (9) and has demonstrated efficacy and safety in reducing the core symptoms of ADHD (9,10). However, although MPH and AHC can effectively manage ADHD symptoms in most pediatric patients, many patients still fail to respond optimally to either.
A number of foreign studies have shown that IR-MPH, OROS-MPH and AHC are effective and well tolerated in children and adolescents with ADHD (11-13). Chinese studies have focused on the effectiveness and safety of IR-MPH, OROS-MPH and AHC for the treatment of ADHD in children and adolescents, particularly IR-MPH. However, it is still controversial as to which of them is the most safe and effective option for ADHD children and adolescents in China. Therefore, the urgent need is to collect the data from clinical setting for comparing the effectiveness and safety of IR-MPH, OROS-MPH and AHC and then provide more supportive evidence for their usage in the clinic practice. In view of this, this systematic review summarized domestic and international published literatures on IR-MPH, OROS-MPH and AHC for Chinese children and adolescents with ADHD to evaluate their effectiveness and safety for informing the administration of these drugs.
Subjects and methods
Search strategy
Relevant publications were retrieved from CNKI, VIP and CBMDICS online using the following keywords or subject terms: Attention deficit hyperactivity disorder or ADHD, Ritalin or methylphenidate or immediate-release methylphenidate hydrochloride, methylphenidate hydrochloride controlled-release or controlled-release methylphenidate, and hydrochloride atomoxetine or atomoxetine. Literature related to Chinese children and adolescents were retrieved from PubMed, Embase and MEDLINE databases using the following subject terms: Methylphenidate, atomoxetine, and attention deficit disorder with hyperactivity. Relevant references were traced. Qualified articles from the earliest to those recorded in September 2010 in each database were used in this study.
Inclusion criteria
An eligible article should: (I) be designed as a randomized controlled trial or controlled clinical trial; (II) enroll Chinese children between 6 and 18 years of old who were diagnosed with ADHD according to the 4th Edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (14), ICD-10, CCMD-3 or CCMD-II-R; (III) aim to compare the effectiveness and safety of IR-MPH, OROS-MPH and AHC, or any two of them; and (IV) have a clear description of the outcomes.
Literature data extraction and quality assessment
Relevant data were extracted from the articles using Epidata 3.1 software. These included the age, sex, baseline comparability, diagnostic criteria, interventions, follow-up time, sample size, outcome assessment indicators, outcome values, types, number and severity of adverse events, Symptom Rating Scale (TEES) scores during treatment, randomization scheme, allocation concealment, whether double-blind, patients lost to follow-up and approaches to outcome analysis.
The quality of an article was assessed in terms of its randomization method, allocation concealment, double-blind design, number of patients lost to follow-up and methods of outcome analysis. The assessment was completed by two independent reviewers. In the case of discrepancies unresolved through discussion, a third reviewer's opinion was sought.
Statistical analysis
The definition of the total incidence of adverse events in this study was different from the typical incidence. The numerator of the total incidence was the adverse events frequency (one patients would be count twice if he suffered two kinds adverse events) and the denominator was the number of patients. Analysis of included literature was completed in EXCEL 4.0 software.
Results
Characteristics of included literature
Of 89 retrieved articles, eight were included in the final analysis based on the inclusion criteria. Four articles compared IR-MPH and OROS-MPH in terms of their effectiveness and safety while the other four compared IR-MPH and AHC. None of the included articles described the therapeutic effect on comorbid conditions associated with ADHD. They had a small sample size (35 to 80 cases) with good baseline comparability in common, despite different medication doses, follow-up periods and indicators used in outcome evaluation (Table 1).

Full table
Quality evaluation of the articles
Of the eight studies, six had randomization but only one of them used allocation concealment and two were double-blind designs. Six studies did not have patients lost to follow-up, while the other two had such subjects but failed to provide the reasons. Six of them conducted intention-to-treat analysis (ITT) and the other two carried out per-protocol analysis (Table 2).
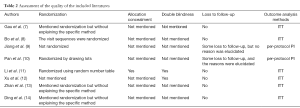
Full table
Evaluation of therapeutic efficacy
OROS-MPH versus IR-MPH
Three out of four studies used PSQ as an outcome indicator, though one of them (15) did not provide the specific values of PSQ. In view of the small number of included articles and discrepancies in outcome indicators (Table 1), only the results of those studies would be described in this section.
In the study conducted by Gau et al. (16), no statistical difference in the decrease of CTRS-R:S, CPRS-R:S and SKAMP scores was observed between the two groups on day 6, 13, 20 and 27 of treatment, though OROS-MPH was associated with faster reduction in the scores of all behavior dimensions than IR-MPH; the SAICA score suggested that OROS-MPH was significantly more effective in improving peer relations than IR-MPH; and the CGI-I score and mother satisfaction were also noticeably higher in the OROS-MPH group than in IR-MPH group. The study by Bo et al. (17) showed no difference in the Conners hyperactivity index score between the two groups. In their study, patients in both groups had significant improvement in all aspects of PSQ scores except the psychosomatic factor in the IR-MPH group. Both groups had a higher C-WISC score after treatment, though the difference was not compared. The difference in the efficacy between the two groups was not significant. Jiang et al. (15) showed PSQ scores in both groups significantly decreased compared with before treatment but did not compare the difference between groups. Pan et al. (18) showed significant differences in the SNAP total scores and sub-scores, and the IVA-CPT scores (except vision control quotient), of both groups compared with before treatment, though the difference in such improvement was not significant between the two groups. However, any mean difference between each value before and after 6-week treatment in the OROS-MPH group was higher than that in the IR-MPH group.
In summary, IR-MPH and OROS-MPH were effective treatment for ADHD. Compared with IR-MPH, OROS-MPH might be better in improving peer relationships, CGI-I score and mother satisfaction, psychosomatic factors, SNAP scale score and the IVA-CPT Rating Scale scores.
AHC versus IR-MPH
Two out of four studies used ADHDRS-IV-Parent:Inv, CGI-S, CPRS-R:S and other indicators for outcome evaluation, and the other two used the Conners hyperactivity index scale. In view of the small number of included articles and discrepancies in outcome indicators, only the results of those studies would be described in this section.
The study of Xu et al. (19) showed significant difference in the ADHDRS-IV-Parent:Inv, CGI-S and CPRS-R:S scores in both groups before and after treatment. Zhan et al. (20) and Ding Airu et al. (21) also showed difference in the Conners hyperactivity index.
In summary, AHC and IR-MPH were effective for ADHD, but there was no difference between them in terms of ADHDRS-IV-Parent:Inv, CGI-S, CPRS-R:S and the Conners hyperactivity index.
Safety evaluation
OROS-MPH versus IR-MPH
Apart from the study of Bu et al. (17) that compared adverse reactions between groups using the TESS score table, the other three (15,16,18) studies reported the number of cases with adverse events in the two groups. As a result, adverse events of those three reports were analyzed in combination.
Among 139 patients treated with OROS-MPH, 108 cases of adverse events were reported and the total incidence was 77.7%. For the 131 patients receiving IP-MPH treatment, 110 cases of adverse events occurred and the total incidence was 84.0%. Adverse events associated with OROS-MPH, in descending order of frequency, were loss of appetite, sleep disorders and stomach pain. As with IR-MPH, the three most common adverse events were loss of appetite, headache and constipation. As shown by the four studies, the adverse events were mild and the incidence rates were not significantly different between the two groups. Gau et al. (16) showed that anxiety, onychophagy and appetite improved more quickly in the OROS-MPH group than in IR - MPH group.
AHC versus IR-MPH
Among 105 patients treated with AHC, 54 cases of adverse reactions were reported and the total incidence was 51.4%. For the 103 patients receiving IP-MPH treatment, 51 cases of adverse reactions occurred and the total incidence was 49.5%. Adverse events associated with AHC, in descending order of frequency, included loss of appetite, sleep disorders and stomach pain. As with IR-MPH, the three most common adverse reactions were loss of appetite, dizziness and abdominal pain. As shown by the four studies, the adverse events were mild and the incidence frequency were not significantly different between the two groups.
Discussion
Due to inconsistent diagnostic criteria, medication dose, follow-up time and other factors that might affect the outcome evaluation, as well as varying indicators for outcome analysis, the included studies were not perfectly eligible for pooled analysis. The small number of included articles also limited the value of the pooled analysis. Hence, this review only provided qualitative description of the study results. The poor quality of a study would affect the validity and reliability of the outcomes when included in a pooled analysis. In this review, the study results were of certain significance because they had good intergroup comparability as shown in Table 1, though the quality and compliance with report standards of related clinical studies should be further improved.
This review suggested that IR-MPH, OROS-MPH and AHC were effective for children and adolescents with ADHD in China. There was no difference in the efficacy ratings across different scales and dimensions between OROS-MPH versus IR-MPH and AHC versus IR-MPH. Compared with IR-MPH, OROS-MPH might be better in improving peer relationships, CGI-I score and mother satisfaction, psychosomatic factors, SNAP scale score and the IVA-CPT Rating Scale. No clinical research comparing the efficacy between OROS-MPH and AHC was found. Steele et al. (22) conducted a study to compare OROS-MPH and IR-MPH, which found that OROS-MPH was superior to IR-MPH in terms of a variety of clinical outcome measures, including the complete remission rate. Xu et al. (23) carried out a meta-analysis of domestic and international randomized, controlled studies on IR-MPH and AHC for ADHD children, finding that they had equivalent total scores regarding the improvement of ADHD conditions, though IR-MPH had better subscores than AHC. This review showed no difference between IR-MPH versus OROS-MPH and IR-MPH versus AHC, which might be due to a small number of included studies, inconsistent follow-up period (mostly not long enough), low quality of included studies and varying definition of adverse events.
As revealed in the two pair of comparisons, the incidence rates of adverse events were similar across groups without significant difference. Since all adverse reactions were mild, the three drugs could be considered safe and well tolerated. Loss of appetite was the most common adverse reaction with the three drugs.
The treatment for ADHD comorbid tic disorder was challenging as 15-30% children with ADHD presented tic symptoms or worsened underlying conditions after administration of stimulants (24). Overseas research showed that AHC not only improved the core symptoms of ADHD remarkably but also reduced the severity of tics in ADHD children with comorbid tic disorders (25,26). Zhang et al. (24) also reported that AHC was significantly effective for hyperactivity, attention deficit, motor tics, vocal tics in ADHD children with comorbid tic disorders. Foreign studies suggested that AHC could be useful for treating ADHD comorbid anxiety (27). However, few studies were done on the treatment of ADHD comorbidity with AHC in China. None of the included studies in this review identified comorbid conditions in their subjects. Hence, well-designed randomized, controlled trials would be needed to compare the effectiveness and safety of AHC, as well as IR-MPH and OROS-MPH for Chinese children and adolescents with ADHD and comorbid conditions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007;164:942-8. [PubMed]
- Buchhorn R, Conzelmann A, Willaschek C, et al. Heart rate variability and methylphenidate in children with ADHD. Atten Defic Hyperact Disord 2012;4:85-91. [PubMed]
- Polderman TJ, Boomsma DI, Bartels M, et al. A systematic review of prospective studies on attention problems and academic achievement. Acta Psychiatr Scand. 2010;122:271-84. [PubMed]
- Taurines R, Schmitt J, Renner T, et al. Developmental comorbidity in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2010;2:267-89. [PubMed]
- Spencer T, Biederman J, Wilens T. Attention-deficit/hyperactivity disorder and comorbidity. Pediatr Clin North Am 1999;46:915-27. [PubMed]
- Renner TJ, Gerlach M, Romanos M, et al. Neurobiology of attention-deficit hyperactivity disorder. Nervenarzt 2008;79:771-81. [PubMed]
- Van der Oord S, Prins PJ, Oosterlaan J, et al. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev 2008;28:783-800. [PubMed]
- Adesman AR. New medications for treatment of children with attention-deficit/hyperactivity disorder: review and commentary. Pediatr Ann 2002;31:514-22. [PubMed]
- Zheng Y, Wang YF, Qin J, et al. Prospective, naturalistic study of open-label OROS methylphenidate treatment in Chinese school-aged children with attention-deficit/hyperactivity disorder. Chin Med J 2011;124:3269-74. [PubMed]
- Pliszka SR, Liotti M, Bailey BY, et al. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2007;17:356-66. [PubMed]
- McGough JJ, McBurnett K, Bukstein O, et al. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2006;16:351-6. [PubMed]
- Kratochvil CJ, Wilens TE, Greenhill LL, et al. Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006;45:919-27. [PubMed]
- Weiss M, Tannock R, Kratochvil C, et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005;44:647-55. [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder, 4th ed. Washington DC: American Psychiatric Association, 1994: 129-30.
- Jiang WQ, Du YS. The efficacy of concerta and ritalin on attention deficit and hyperactivity disorder. Shanghai Archives of Psychiatry 2007;19:25-7.
- Gau SS, Shen HY, Soong WT, et al. An open-label, randomized, active-controlled equivalent trial of osmotic release oral system methylphenidate in children with attention-deficit/hyperactivity disorder in Taiwan. J Child Adolesc Psychopharmacol 2006;16:441-55. [PubMed]
- Bu R, Zhou HH, Sun JR, et al. A comparison study between two types of methylphenidate hydrochloride tablets in the treatment of attention deficit and hyperactivity disorder. J Clin Psychiatry 2008;118:381-3.
- Pan XX, Ma HW, Wan B, et al. Effectiveness of oral osmotic-methylphenidate in treatment of attention deficit hyperactivity disorder in children. Zhongguo Dang Dai Er Ke Za Zhi 2008;10:471-4. [PubMed]
- Xu T, Zhou Y, Wei HW, et al. Comparison of atomoxetine and methylphenidate for treating children with attention deficit hyperactivity disorder. Chinese Journal of Practical Pediatrics 2008;23:499-501.
- Zhan XM, Zhou YF, Zou XH. Clinical controlled study of childhood Hyperkinetic Syndrome with Tomoxetine and Ritalin Treatment. China Pharmaceuticals 2009;18:72-3.
- Ding AR, Ye JF, Zhou YF. Curative efficacy of Tomoxetine in the treatment of childhood hyperkinetic syndrome. Strait Pharmaceutical Journal 2009;21:121-2.
- Steele M, Weiss M, Swanson J, et al. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can J Clin Pharmacol 2006;13:e50-62. [PubMed]
- Xu PR, Fang ZM. A Meta-analyses Comparing Atomoxetine with Methylphenidate for Treatement of Children with Attention-Deficit/Hyperactivity Disorder. Chin J Evid-based Med 2009;9:346-9.
- Zhang YJ, Wang YF. Therapeutic Effects of Atomoxetine on Children with Attention Deficit Hyperactivity Disorder Comorbid Tic Disorder. J Appl Clin Pediatr 2009;24:220-2.
- Spencer TJ, Sallee FR, Gilbert DL, et al. Atomoxetine treatment of ADHD in children with comorbid Tourette syndrome. J Atten Disord 2008;11:470-81. [PubMed]
- Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005;65:1941-9. [PubMed]
- Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27. [PubMed]


