
An email-based survey of practice regarding hemodynamic monitoring and management in children with septic shock in China
Introduction
Hemodynamic monitoring (HM) remains an important aspect of critically ill patient care, which originally promoted by Rivers et al. for patients with severe sepsis and septic shock (1). Although the use of HM at the bedside faces many challenges (2,3), goal-directed strategies of hemodynamic management based on parameters of extended hemodynamic and metabolic monitoring are increasingly recommended in different national and international guidelines (4-7).
How HM is actually practiced in intensive care units (ICU) has been described by surveys in many countries including Swiss (2,7), Austrian (7,8), Italian (9,10), the United Kingdom (11), USA (12), Brazil (13), France (14,15), German (7,16) and fields (10,12,17,18). To date there is little information about practice of HM in pediatric intensive care units in China.
This email-based survey aimed to study the current situation regarding the utilization of HM devices, the parameters used in HM, and potential individual and hospital differences in China. We present the following article in accordance with the SURGE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-374).
Methods
Survey study and population
We conducted a prospective, multicenter survey in Chinese pediatric intensive care units (ICU) in 2017. The survey was reviewed and approved by the sub-association of pediatric intensive care physicians, Chinese Medical Doctor Association. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the sub-association of pediatric intensive care physicians, Chinese Medical Doctor Association and Shanghai Children’s Medical Center (No. SCMCIRB-K2015039) and individual consent for this study was waived.
The structured questionnaire was discussed by experts of the sub-association of pediatric intensive care physicians and was finalized after the pretesting in 5 pediatric intensivists from Shanghai Children’s Medical Center, Shanghai. Face validity, content validity, and clinical sensibility were evaluated.
The structure of the survey included a variety of single choice, multiple choice and open-ended questions. None of the questionnaires used a scoring scale. It was designed to take 10-20 minutes to complete. The questionnaire (Appendix 1) consisted of four pages and 22 questions divided in three sections: (I) general information on the hospitals, respective ICUs and participants, (II) the availability of technical equipment and parameters of HM and (III) simulated management of septic shock in three clinical case vignettes. The questionnaire was in Chinese.
National pediatric intensivists were invited to answer an electronic questionnaire at the second academic conference held in Beijing in 2017. We directly contacted members of the aforementioned association via email during a 2-week period from 1–15 May 2017. A cover letter (Appendix 2) explained the background, aims, and methods of the study and requested E-mail addresses of all pediatric intensivists in the member’s units. Questionnaire sheets were mailed to all participants on 16–30th May 2017, and the data were collected over a 4-weeek period, which was closed end of June 2017. Responses were voluntary, anonymous and unpaid. If the survey questionnaire was not recovered within the 4-week recycling period, a reminder would be sent by phone or Email.
In the study, junior doctors were young faculty members. Basic HM included electro-cardiogram, peripheral oxygen saturation, blood pressure (invasive and non-invasive), blood lactic acid level, capillary refill time (CRT) and urine output measurement. Advanced HM included central venous pressure (CVP), cardiac output indicators [Cardiac output (CO), Cardiac output index (CI), left ventricular ejection fraction (LVEF), stroke volume (SV), and Velocity time integral of subaortic blood flow (VTI)], Central venous oxygen saturation (ScvO2), fluid responsiveness and volume status (FR-VS) indicators [stroke volume variation (SVV), pulse pressure variation (PPV), inferior vena cava variation (IVC), and passive leg rising (PLR)], systemic vascular resistance (SVR) and index (SVRI), PCO2 gap between central venous and artery (Pcv-aCO2), extravascular lung water index (EVLWI), and tissue O2 pressure/tissue CO2 pressure (PtO2/PtCO2). All advanced HM were obtained from non-invasive and/or invasive methods. For example, CO/CI was available from transpulmonary thermodilution and/or bedside ultrasound technology led by intensivists. Bioreactance and ultrasound technology were non-invasive HM methods and transpulmonary thermodilution was an invasive procedure.
Statistical analyses
Data analysis was performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Demographic characteristics were summarized by proportions for categorical data. For descriptive statistical analysis, we calculated absolute and relative frequencies (in percentage) to describe categorical data. Categorical variables were analyzed using the chi-square test. All statistical tests were two-sided, and statistical significance was defined as a P value of less than 0.05. Multivariable analyses to identify demographic and professional characteristics associated with the dependent outcomes were performed using binary logistic regression.
According to the questionnaire sample scale, we calculated that 384 respondents were needed, with a sampling error of 5% and a confidence interval of 95%. We estimated that a study period of 4 weeks would be necessary to recover at least 384 questionnaires and reduce the non-response error. Results were analyzed according to the number of responses for each given question. The numerator and denominator for the prevalence calculation came from the survey form. The denominator of the response-rate was the number of emails we had collected and successfully sent. Reply to email and completion of any portion of the survey implied consent to participate. Ten hospitals did not submit any form and 17 people ended the survey incompletely and thus were excluded. There was no missing data.
Results
Response rate
Responses were obtained from 385 of 854 pediatric intensivists (response-rate 45.1%) emailed and 68 of 78 hospitals (response-rate 87.2%) in total. Seventeen people ended the survey incompletely, resulting in 368 fully completed responses. Fifteen questionnaires from the two hospitals were identical, and the other two questionnaires lacked more than 50% of the items, so they were excluded.
Personal and practice characteristics
Tables 1 and 2 showed the demographic characteristics of 68 hospitals and 368 respondents. The health care sites at which respondents primarily practiced were mainly tertiary hospitals (94.1%) and general pediatric ICUs (85.3%), but less ICU specialist training centers (39.7%). The majority of respondents had master degree (58.7%) and intermediate professional title (37.2%), and was aged 30–40 (55.7%), 65.2% respondents reported managing more than 30 cases of septic shock within 1 year. 60.9% respondents stated attending the pediatric advanced life support (PALS) course.
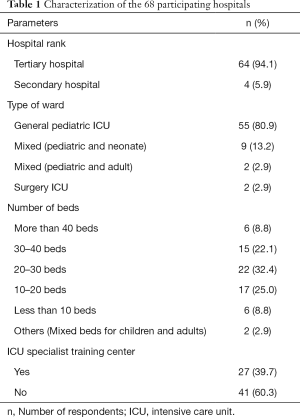
Full table
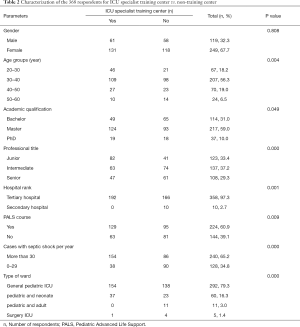
Full table
Parameters of HM
Table 3 presents the most available variables of HM. Basic HM (93–100%) were reported as the most utilized parameters, 81.6% (279/342) of the respondents reported starting urine volume measurement within 1 hour of admission, 3.5% (12/342) within 3 hours, and 14.9% (51/342) within 6 hours.
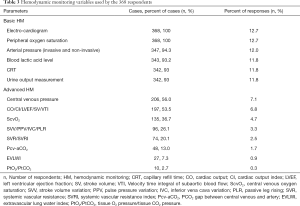
Full table
Reported advanced HM were variable. The most frequently advanced HM included CVP (56.0%), CO/CI/LVEF/SV/VTI (53.5%), and ScvO2 (36.7%). The least reported HM was PtO2/PtCO2 (2.7%).
Utilization of non-invasive advanced HM devices and factors associated with the use of non-invasive advanced HM devices by the 368 respondents, l,225 respondents (225/368, 61.1%) stated that non-invasive advanced monitoring equipment could be used in their hospitals. Three devices were most commonly available: bedside echocardiography led by intensivists (164/368, 44.6%), ultrasound cardiac output monitor (USCOM) (68/368, 18.5%), and bioreactance (NICOM) (69/368, 18.8%). Respondents were divided into two groups according to the utilization of non-invasive HM equipment: yes (n=225) or no (n=143). Table 4 showed that there were significant differences in academic qualification (P=0.011), number of patients with septic shock per year (P=0.000) and staff of ICU specialist training center (P=0.000) according to the utilization of HM devices. Multivariable analyses identified factors associated with the utilization of HM devices (Table 5). More than 30 cases with septic shock per year (P=0.002) and staff of ICU specialist training center (P=0.003) were associated with higher utilization of the devices.
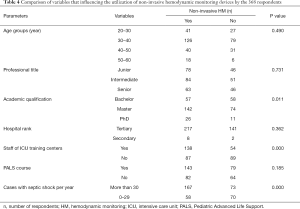
Full table

Full table
FR-VS assessment in the clinical case vignette and factors associated with FR-VS assessment
We performed a survey study in which the respondents responded to the simulated management of three clinical vignettes with septic shock. 49.7% (183/368) of respondents reported conducting FR-VS assessment. Instruments used in FR-VS assessment include bedside echocardiography led by intensivists [39.4% (145/368)], NICOM [10.3% (38/368)], transpulmonary thermodilution devices [6.3% (23/368)]; 4.9% (18/368) of respondents reported using both non-invasive HM devices, while 6.0% (22/368) reported using both non-invasive and invasive HM devices. Compared with the number of respondents who report using non-invasive instruments in their hospitals, 88.4% (145/164) of respondents reported using bedside echocardiography in the simulated clinical vignettes and 55.1% (38/69) reported using NICOM, with no mention of USCOM.
Table 6 showed that starting FR-VS assessment did not differ regarding age, professional title, hospital rank, PALS course or fluid resuscitation decision. Multivariable analyses identified factors associated with FR-VS assessment (Table 7). FR-VS assessment was associated with high academic qualification (P=0.030) and staff of ICU specialist training center (P=0.005).
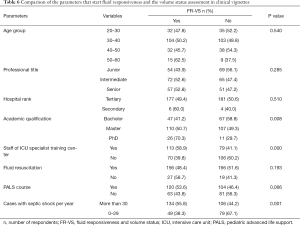
Full table

Full table
Discussion
Based on E-mail survey, this study evaluated the practice regarding basic and advanced HM and management in children with septic shock in China.
With no doubt, the survey showed that almost every patient with septic shock performed basic HM, which was similar to the study by Saugel et al. (19) and Funcke et al. (7). It is encouraging to note that the proportion of urine output measurement (81.6%) in 1-hour in our survey was significantly higher than that (68.1%) in the survey conducted from May 2011 to January 2012 in three PICUs of tertiary teaching hospitals in Shanghai, China (20).
Extended HM parameters and in particular monitoring of cardiac output (CO/CI/LVEF/SV/VTI) and ScvO2, were used all over in less than 50% of the respondents, which was similar to that in Saugel’s and Biancofiore’s studies (9,19), but higher than that in Funcke’s report (only 24% of all ICU patients’ CO monitoring based on individual patient data) (7). These findings might indicate that the pediatricians in our survey (mainly pediatric intensivists as primary specialty), compared with the anesthesiologists in the study of Funcke et al. (7), performed CO/CI/LVEF/SV/VTI monitoring more often, or that the answers given by the pediatricians in our survey (that was not based on individual patient data) overestimate the actual proportion of patients undergoing CO monitoring.
Less than 5% of the respondents reported using advanced hemodynamic variables of microcirculation, such as PtO2 and PtCO2, which was similar to the study of Funcke et al. (7). Whether the low frequency of use was caused by a lack of confidence in monitoring accuracy or by other reasons (for example cognitive, economic) was beyond the scope of the survey.
In our survey CVP was used for preload monitoring in almost half of the respondents and the use of volumetric or dynamic parameters of preload was infrequent (25% of the respondents), which was similar with earlier data from Funcke et al., Cecconi et al. and Preau et al. (7,11,14). However, the poor use of these predictive indices observed in our survey was in contrast with their frequent use (49.7%) in the clinical case vignette, with the major limitation inherent to studies collecting declarative data. In addition, the significant use limitation of some parameters such as PLR in children may also account for the low frequency of use. This also illustrated the gap among theoretical, physiological knowledge and routine practice (15).
The most frequent invasive extended hemodynamic technology was transpulmonary thermodilution (21), however, the actual use of this monitoring modality for fluid therapy in the clinical case vignette with septic shock was comparably low (6.3% of respondents) in our study. In contrast, non-invasive HM devices were frequently used (61.1% of respondents reported using in their hospitals and 49.7% of respondents reported using in the clinical case vignettes), which was similar to the earlier data from Italian (9). Bedside transthoracic echocardiography led by intensivists was the most commonly used device in the survey. Our study suggests that there may be an increasing awareness and acceptance of non-invasive HM in pediatric intensive care medicine (outside cardiac surgery) in China. However, several studies have reported the gap between the high availability (7,11,15) or the strong recommendations (22,23) of noninvasive HM and their actual low clinical use in patients, which were out of our declarative data description. Of note, there were obviously differences between staff of ICU training centers and sepsis numbers per year regarding utilization of these noninvasive technologies in this survey. Our survey may suggest that professional training platform and the number of septic shock admission per year were major factors in the introduction and use of new technologies. Boulain et al. (15) also had reported the between-center heterogeneity in HM in 19 French ICU.
Clinical case vignettes were designed to indirectly assess the practical application of advanced HM in the respondents. The survey found that FR-VS assessment was not related to the volume expansion decision, although staff in training centers and highly educated individuals tended to assess the hemodynamics. Cecconi et al. (11) reported that patients in the FENICE Study received further fluids despite no response to the initial fluid challenge. Preau et al. (14) reported that dynamic parameters were often incorrectly used in the presence of contraindications in six French ICU. Those findings highlight the great variability in fluid management in critically ill patients, the gap in integrating different hemodynamic variables into management decisions, and the importance of continuing education and training of advanced HM at different levels and in different ways (13,16).
Our study has several strengths. We received responses from multiple different regions in China, ranging from high-income to lower middle-income regions. Respondents were PICU specialists at different professional title and academic qualification, and mainly working in tertiary hospitals.
Our survey has the limitations of being addressed only to the members of Association of Pediatric Intensivists who responded in a limited number. Therefore, it may only mirror the attitude and practice of Chinese pediatric intensivists in using HM to a certain extent. It is also possible that there was a selection bias, as pediatricians interested in hemodynamics may have greater tendency to being willing to answer the questionnaire. We were unable to determine causality or potential direction of effect for the associations observed through an email-based survey. Second, even if we consider that our survey results represent the actual practice of HM in critically ill children in China three years ago, but the knowledge and education of advanced HM, especially non-invasive HM, have been greatly improved in pediatrics in recent years. Third, to describe the degree of the simulated management of HM, a simple method (case vignette) was used to assess the consensus on clinicians’ practices. Finally, given the inherent flaws in self-reported survey research, the gap between perception of practice and the real-life practice at the bedside may be significant. A multicenter study either prospective or retrospective data review would be a stronger study to find out an actual prevalence of these monitoring in real practice scenarios.
In conclusion, there was a large variability in use advanced HM parameters. Almost half of the respondents reported to use advanced HM such as CVP, cardiac output indicators (CO/CI/LVEF/SV/VTI) and ScvO2, but the use of volumetric or dynamic indices of preload and microcirculation was infrequent in our study. There was a growing awareness and acceptance of non-invasive HM, but FR-VS assessment was not related to volume expansion decision. There was a potential need for hemodynamic education and training in pediatric intensive care medicine (outside cardiac surgery) in China.
Acknowledgments
We thank the sub-association of pediatric intensive care physicians, Chinese Medical Doctor Association for the support of this survey. We are also very grateful to all respondents and PICUs for their participation (in Chinese Pinyin order): Anhui Provincial Children’s Hospital Anhui Provincial Hospital, Bethune First Hospital Affiliated to Jilin University (Jilin Province), Beijing Children’s Hospital, Capital Medical University (Beijing), Changzhou Children’s Hospital (Jiangsu Province), Chendu Women’s and Children Central Hospital (Sichuan Province), Dalian Children’s Hospital Affiliated to Dalian Medical University (Liaoning Province), The Second Hospital of Dalian Medical University (Liaoning Province), Dongying People’s Hospital (Shandong Province), Ezhou Central Hospital (Hubei Province), Children’s Hospital of Fudan University (Shanghai), Shanghai Children’s Medical Center affiliated to School of Medicine, Shanghai Jiaotong University (Shanghai), Foshan Women and Children Hospital (Guangdong Province), Gansu Provincial Maternity and Child-care Hospital (Gansu Province), Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Guangzhou Women and Children’s Medical Center (Guangdong Province), Maternal and Child Health Hospital of Guiyang (Guizou Province), Harbin Children’s Hospital (Heilongjiang Province), First Affiliated Hospital of Harbin Medical University (Heilongjiang Province), Children’s Hospital of Hebei Province, The Fourth Hospital of Hebei Medical University (Hebei Province), Sanmenxia Central Hospital (Henan Province), Xihua County People’s Hospital (Zhoukou City, Henan Province), Maternal and Child Health Hospital of Hubei Province, Hunan Children’s Hospital (Hunan Province), Hunan Provincial People’s Hospital (Hunan Province), Tongji Hospital Affiliated to Huazhong University of Science and Technology (Hubei Province), Wuhan Union Hospital Affiliated to Huazhong University of Science and Technology (Hubei Province), The People’s Hospital of Jianyang (Sichuan Province), Kunming Children’s Hospital (Yunnan Province), The First Hospital of Lanzhou University (Gansu Province), Maternal and Child Health Hospital of Linyi (Shandong Province), Children’s Hospital of Nanjing Medical University (Jiangsu Province), Nanyang Central Hospital (Henan Province), Affiliated Hospital of Inner Mongolia Medical University (Inner Mongolia Autonomous Region), Maternal and Child Health Hospital of Inner Mongolia Autonomous Region; Puyang People’s Hospital (Henan Province), Maternal and Child Health Hospital of Qinghai Province; Quanzhou First Hospital (Fujian Province), The first Affiliated Hospital of Xiamen University (Fujian Province), Shandong Provincial Hospital, Qilu Children’s Hospital of Shandong University (Shandong Province), Maternal and Child Health Hospital of Taian (Shandong Province), Children’s Hospital of Shanghai affiliated to Shanghai Jiaotong University (Shanghai), Maternal and Child Health Hospital of Bao ‘an District (Shenzhen, Guangdong Province), Shenzhen Children’s Hospital (Guangdong Province), Shengjing Hospital of China Medical University (Liaoning Province), Taihe Hospital (Shiyan City, Hubei Province), First Affiliated Hospital, School of Medicine, Shihezi University (Xinjiang Uygur Autonomous Region), Children’s hospital affiliated to Capital Institute of Pediatrics (Beijing), Maternal and Child Health Hospital of Sichuan Province; Sichuan Provincial People’s Hospital, Tianjin Children’s Hospital (Tianjin); Wuhan Children Hospital Affiliated to Huazhong University of Science and Technology (Hubei Province), Xi’an Children’s Hospital (Shaanxi Province), Xianyang Rainbow Hospital (Shaanxi Province), Xinhua Hospital affiliated to School of Medicine, Shanghai Jiaotong University (Shanghai), People’s Hospital of Xinjiang Uygur Autonomous Region, Xuzhou Children’s Hospital (Jiangsu Province), Changchun Children’s Hospital (Jilin Province), Children’s Hospital Affiliated to Medical College of Zhejiang University (Zhejiang Province), The Third Affiliated Hospital of Zhengzhou University (Henan Province), The First Affiliated Hospital of Zhengzhou University (Henan Province), Zhengzhou Children’s Hospital (Henan Province), First Affiliated Hospital of Sun yat-sen University (Guangdong Province), Children’s Hospital of Chongqing Medical University (Chongqing), Zhujiang Hospital of Southern Medical University (Guangdong Province), Affiliated Hospital of Zunyi Medical University (Guizhou Province).
Funding: This work was supported by Pudong new area science and technology development (PKJ2015-Y06), Pudong new area, Shanghai, China.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-374
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-374
Peer Review File: Available at http://dx.doi.org/10.21037/tp-20-374
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-374). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the sub-association of pediatric intensive care physicians, Chinese Medical Doctor Association and Shanghai Children’s Medical Center (No. SCMCIRB-K2015039) and individual consent for this study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Siegenthaler N, Giraud R, Saxer T, et al. Haemodynamic monitoring in the intensive care unit: results from a web-based Swiss survey. Biomed Res Int 2014;2014:129593 [Crossref] [PubMed]
- De Backer D. Detailing the cardiovascular profile in shock patients. Critical Care 2017;21:311. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Habicher M, Zajonz T, Heringlake M, et al. S3 guidelines for intensive care in cardiac surgery patients: hemodynamic monitoring and cardiocirculatory system-an update. Anaesthesist 2018;67:375-9. [Crossref] [PubMed]
- Funcke S, Sander M, Goepfert MS, et al. Practice of hemodynamic monitoring and management in German, Austrian, and Swiss intensive care units: the multicenter cross-sectional ICU-CardioMan Study. Ann Intensive Care 2016;6:49. [Crossref] [PubMed]
- Menger J, Edlinger-Stanger M, Dworschak M, et al. Postoperative Management of Patients Undergoing Cardiac Surgery in Austria: A National Survey on Current Clinical Practice in Hemodynamic Monitoring and Postoperative Management. Wien Klin Wochenschr 2018;130:716-21. [Crossref] [PubMed]
- Biancofiore G, Cecconi M, Rocca GD. A Web-Based Italian Survey of Current Trends, Habits and Beliefs in Hemodynamic Monitoring and Management. J Clin Monit Comput 2015;29:635-42. [Crossref] [PubMed]
- Rizza A, Bignami E, Belletti A, et al. Vasoactive Drugs and Hemodynamic Monitoring in Pediatric Cardiac Intensive Care: An Italian Survey. World J Pediatr Congenit Heart Surg 2016;7:25-31. [Crossref] [PubMed]
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 2015;41:1529-37. Erratum in: Intensive Care Med 2015;41:1737-8. [Crossref] [PubMed]
- Cannesson M, Pestel G, Ricks C, et al. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Critical Care 2011;15:R197. [Crossref] [PubMed]
- Dias FS, Rezende EAC, Mendes CL, et al. Hemodynamic monitoring in the intensive care unit: a Brazilian perspective. Rev Bras Ter Intensiva 2014;26:360-6. [Crossref] [PubMed]
- Preau S, Dewavrin F, Demaeght V, et al. The use of static and dynamic haemodynamic parameters before volume expansion: A prospective observational study in six French intensive care units. Anaesth Crit Care Pain Med 2016;35:93-102. [Crossref] [PubMed]
- Boulain T, Boisrame-Helms J, Ehrmann S, et al. Volume expansion in the first 4 days of shock: a prospective multicentre study in 19 French intensive care units. Intensive Care Med 2015;41:248-56. [Crossref] [PubMed]
- Saugel B, Reese PC, Wagner JY, et al. Advanced hemodynamic monitoring in intensive care medicine: A German web-based survey study. Med Klin Intensivmed Notfmed 2018;113:192-201. [Crossref] [PubMed]
- Hanot J, Dingankar AR, Sivarajan VB, et al. Fluid Management Practices After Surgery for Congenital Heart Disease: A Worldwide Survey. Pediatr Crit Care Med 2019;20:357-64. [Crossref] [PubMed]
- Conlon TW, Kantor DB, Su ER, et al. Diagnostic Bedside Ultrasound Program Development in Pediatric Critical Care Medicine: Results of a National Survey. Pediatr Crit Care Med 2018;19:e561-8. [Crossref] [PubMed]
- Saugel B, Huber W, Nierhaus A, et al. Advanced Hemodynamic Management in Patients With Septic Shock. Biomed Res Int 2016;2016:8268569 [Crossref] [PubMed]
- Qian J, Wang Y, Zhang Y, et al. A Survey of the First-Hour Basic Care Tasks of Severe Sepsis and Septic Shock in Pediatric Patients and an Evaluation of Medical Simulation on Improving the Compliance of the Tasks. J Emerg Med 2016;50:239-45. [Crossref] [PubMed]
- Jain M, Canham M, Upadhyay D, et al. Variability in interventions with pulmonary artery catheter data. Intensive Care Med 2003;29:2059-62. [Crossref] [PubMed]
- Expert Round Table on Echocardiography in ICU. International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med 2014;40:654-66. [Crossref] [PubMed]
- Via G, Hussain A, Wells M, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014;27:683.e1-33. [Crossref] [PubMed]


