
Clinical analysis of seven pediatric patients with coronavirus disease 2019 (COVID-19) in Jingzhou, Hubei, China: a retrospective study
Introduction
Since December 2019, the novel Coronavirus disease 2019 (COVID-19) epidemic has progressed into a worldwide pandemic. Investigations revealed that the virus had mutated into a novel coronavirus, and the International Committee on Virus Classification named it “sudden acute respiratory syndrome (SARS)-COV-2”, as the coronavirus has been internationally recognized to cause acute respiratory syndrome (1). The World Health Organization (WHO) named the novel coronavirus pneumonia “CoVID-19”. The virus is spread through respiratory droplets and physical contact. During the nascence of the coronavirus outbreak, although all people were susceptible to SARS-COV-2, the coronavirus infection appeared to be less frequent and severe in children than in adults, which contributed to the idea that children are less sensitive to the coronavirus. Nevertheless, with the emergence of cluster infections in families, reports of childhood illness continue to increase (2-4). In addition, newborn babies of mothers infected with novel coronavirus were noted. Children are often vulnerable to upper respiratory infections and their immune systems are not fully developed, and the delay of reports about children infected with COVID-19 has been confusing (5). In addition, the low detection rate of coronavirus nucleic acid in throat swabs has made distinguishing coronavirus from other common respiratory infection pathogens in children difficult. It is important to ascertain whether infected children have the same disease progression as adults. Due to the strong measures of control, the epidemic in China has been basically contained. As the coronavirus variant that has moved through the UK is now spreading "rapidly" through the US, the possibility of a change in spectrum of pediatric disease should also be kept in mind (6). At the same time, although the chances of a second outbreak in china are remote, vigilance must be maintained, and a summary of the experience derived from the previous epidemic prevention and control work should be created. In order to provide a reference for resolution of future clinical problems, we retrospectively analyzed the clinical data of 7 children with novel coronavirus infection hospitalized in the Pediatrics Department of The First People’s Hospital of Jingzhou, as diagnosed by laboratory examination. We present the following article in accordance with the AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/tp-21-48).
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The First People’s Hospital of Jingzhou (NO.:20201006). Written consent was provided by the guardians of all enrolled children.
Chest CT and biological test
Chest CT without intravenous contrast was performed on all patients using a Siemens SOMATOM Definition AS128 or GE Optima CT 660 with a 1- or 0.625-mm slice thickness, respectively. Children under 5 years old, as well as uncooperative children, received oral chloral hydrate sedation (0.5 mL/kg) prior to CT. Cooperative children above 5 years old were trained with breathing exercises prior to CT. All CT images were reviewed by at least two radiologists with more than 10 years of experience. Imaging was reviewed independently.
Biological test includes blood routine blood, urine routines, routine stool test, liver function, kidney function, electrolytes, coagulation function, C-reactive protein, lactate dehydrogenase, MB isoenzyme of creatine kinase, mycoplasma pneumoniae and influenza B virus infection.
Study design and participant selection
We retrospectively analyzed children who visited the department of pediatrics fever clinic at The First People’s Hospital of Jingzhou between January 30 and February 29 in 2020. By collecting pharyngeal or anal swab samples from children, real-time quantitative polymerase chain reaction (qPCR) was used to detect SARS-COV-2 virus. The diagnosis, clinical classification, and discharge criteria were based on the Scheme for Diagnosis and Treatment of 2019 Novel Coronavirus Pneumonia (The 7th Trial Edition) which was made by the Chinese National Health Commission (NHC) (7).
Data collection and analysis
A total of 7 children infected with novel Coronavirus were confirmed by pharyngeal swab nucleic acid testing. We retrospectively analyzed the demographic information, epidemiological characteristics, clinical symptoms, laboratory examination, imaging data, treatment, and outcome of the 7 children.
Statistical analysis
The software SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Continuity variables were described as median and interval, and categorical variables were expressed as a count or (and) percentage.
Results
Demographic and epidemiological characteristics
Among the 7 participants study, 2 (29%) were male and 5 (71%) were female, ranging in age from 3 months and 14 days to 12 years (median age, 3 years). All of the children had a history of living in or contact with someone recently returned from Wuhan. In addition, all participants had 2 or more family members with confirmed infection, and there was familial aggregation infection (Table 1).

Full table
Clinical classification and symptoms
Among the 7 children, 1 infection was asymptomatic (14%), 5 were mild infections (71%), and 1 was a moderate infection (14%). The most common symptoms were fever (5/6, 83%) and cough (5/6, 83%). Among the 5 children with fever, low and moderate fever were common (4/5, 80%), 1 case had experienced a high fever, and the highest temperature was 39.8 °C. The 5 children with cough predominantly had occasional cough (4/5, 80%); 1 participant had persistent cough, but none of them had tachypnea or dyspnea. Other symptoms included diarrhea (1/7), eczema (1/7), pharyngalgia (1/7), sneezing (1/7), and runny nose (1/7) (Table 1).
Laboratory test and radiology results
Blood tests and chest computed tomography (CT) were performed at admission or the following day. Most participants (5/7, 71%) had normal white blood cell counts, 1 case was increased (>12.0×109/L) and 1 case was decreased (<5.0×109/L); in 1 case neutrophils were elevated (>6.3×109/L); 4 cases had abnormal lymphocyte counts, which were increased in 3 cases (>3.2×109/L) and decreased in 1 (<1.1×109/L). Liver function results showed an increase in transaminase [alanine aminotransferase (ALT)/aspartate aminotransferase (AST) >40 U/L] in 1 child. Lactate dehydrogenase (LDH) was elevated in 3 participants (>240 U/L). The MB isoenzyme of creatine kinase (CK-MB) was increased in 2 children (>25 U/L). C-reactive protein (CRP) was raised in 2 participants (>8 mg/L). Mycoplasma pneumoniae infection was detected in 1 participant, and 3 children had been complicated by Mycoplasma pneumoniae and influenza B virus infection (Table 2). The renal function, electrolytes, and blood glucose of every child were within the normal ranges. All participants had undergone chest CT scans, from which 1 child was diagnosed with viral pneumonia, showing ground glass opacity in the upper and lower lobes of the left lung (Figure 1); the chest CT scans of the remaining 6 participants were normal.
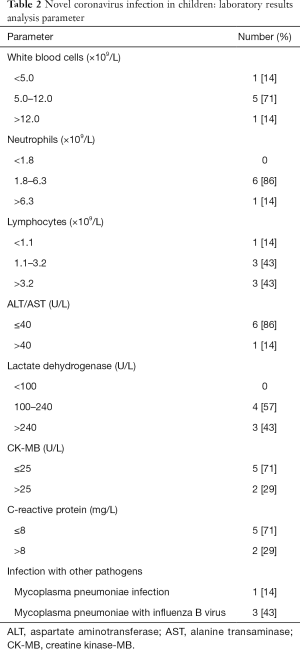
Full table
Treatment and outcomes
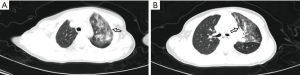
All participants were observed under isolation and administered with antiviral therapy as follows: interferon atomization (7/7), Lianhua Qingwen granules (6/7), abidol (5/7), traditional Chinese medicine (TCM) (4/7), oseltamivir (3/7) for oral treatment. Azithromycin was additionally administered to those with Mycoplasma pneumoniae. The fevers of all febrile children had subsided by the day after admission. During hospitalization, the symptoms of children with occasional cough were relieved, and even completely resolved. The persistent cough of 1 participant had transformed to an occasional single cough after 7−10 days of hospitalization. Diarrhea in 1 participant was treated with probiotics, and the symptoms of diarrhea disappeared 6−7 days after treatment. After treatment with inosine injection and reduced glutathione injection, transaminase decreased continuously in 1 participant who had displayed abnormal liver function. The 2 cases of elevated CRP returned to normal after treatment with antimicrobials. Orally administered Leucoson led to recovery of normal white blood cell count in 1 participant who had displayed previously low levels. A child with an abnormal chest CT was retested on day 7 after admission. The repeat chest CT revealed that some lesions were smaller than previously, and some lesions had virtually absorbed, which was considered an improvement; an additional repeat chest CT on the 27th day after admission showed that the lesions had fully absorbed, and no significantly abnormal lung CT scan findings remained (Figure 2). The median time of nucleic acid testing in throat swabs to become negative was 14 days (6–26 days). A single participant returned persistently positive anal swab nucleic acid test results, but had no other clinical symptoms, and the result became negative almost 2 months after discharge. The median length of stay was 15 days (8–31 days) (Table 3). All of the children were cured and subsequently discharged from hospital.
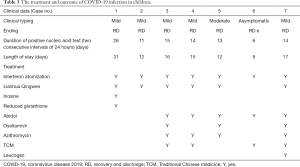
Full table
Discussion
SARS-CoV-2 seem to less commonly affect children and to cause fewer symptoms and less severe disease in this age group compared with adults, and are associated with much lower case-fatality rates. Reports of infections among children have increased along with improvements in testing techniques during the outbreak of COVID-19. According to the Chinese center for disease control and prevention, among more than 72,000 cases reported nationally, about 2.1% were under the age of 19. In the United States, 1.7% of cases of COVID-19 infection of known age occurred in patients younger than 18 years old (8). However, there is limited data on the epidemiological and clinical characteristics of children infected with COVID-19.
In this study, we retrospectively analyzed the clinical characteristics of 7 pediatric patients with COVID-19 infection aged from 3 months to 12 years old in Jingzhou. All participants had family members (2 or more) who were also infected with COVID-19, which indicated familial clustering. According to the Chinese national prevention and control policies, avoiding public places and social gatherings can effectively reduce the overall rate of infection, but as children are dependent on their parents for care, close contact within families may be an important route of transmission of COVID-19 for children in Jingzhou which is similar to SARS and Middle East respiratory syndrome (MERS). The prevention and control of childhood infections depends on cohesive family efforts. Wearing masks, proper hand hygiene, and surface disinfection are simple but important precautions for infection control. Our study included 1 child with asymptomatic infection, which indicated that routine nucleic acid testing of children in families with confirmed or suspected members may be a feasible method of identifying asymptomatic carriers. The remaining 6 children infected with COVID-19 expressed mild symptoms, 5 were mild types, and 1 was a common type. There was no incidence of dyspnea or severe extrapulmonary manifestations during the course of disease, and no one was treated in the intensive care unit (ICU). Fever has subsided in all participants by the day after admission, and all other symptoms were relieved within 7 days. Fever and cough were the main symptoms among participants, which was similar to most other studies, and 1 child with common type exhibited high fever (39.8 °C) and persistent cough. Additionally, a solitary participant, who was 3-month-old girl, had diarrhea, while in other studies, the incidence of diarrhea was 3.7%. Another retrospective analysis of 138 patients showed that about 10% of participants had diarrhea (9,10). The chest CT findings of infected children were mostly mild, which was similar to those of adults (11,12). The typical appearance of chest CT was either unilateral or bilateral subpleural ground-glass shadows with surrounding halos. Although focal absorption on the chest CT consistently lagged behind the improvement of clinical symptoms and nucleic acid tests having turned to negative, the chest CT abnormalities and clinical symptoms did not worsen, which indicated that COVID-19 infection in children is an asymptomatic or mild clinical disease with a good prognosis.
Leukopenia and lymphocytosis were uncommon in pediatric patients with COVID-19. However, up to 83.2% of adults infected with COVID-19 had lymphocytosis (10,13,14), and 33.7% had leukocytosis (13). In our study, only 1 child had developed leukopenia and lymphocytosis during hospitalization, and they quickly returned to normal after treatment. Contrastingly, in our study, lymphocytes were increased in 3 children. This difference may have been due to variations between the immune responses of children and the adults to COVID-19 infection. Most adults expressed significant lymphocytosis, and those who finally died showed severe lymphocytosis counts. For children with mild symptoms and good prognosis in this study, lymphocytes were either normal or increased. Lymphocytosis reflects the depletion of lymphocytes by SARS-COV-2 and indicates serious disease (10,13,14). This suggests that lymphocyte count may be associated with disease severity and prognosis in the patients with COVID-19 infection. A study showed that 86% of adults had varying degrees of increase in C-reactive protein, ALT, AST, and LDH (13), especially in severe and critically ill patients (10,13,14). Such a high proportion of abnormal indicators was not detected in the children in our study, and these abnormal indicators were all slightly biased. In addition, there were 4 children with Mycoplasma pneumoniae or/and influenza B virus infection, but neither of these co-infections compounded the primary disease, which indicated that children with COVID-19 and other common respiratory pathogens infection did not display a worsening trend of disease. As a result, laboratory tests of children infected with COVID-19 did not yield specific results or enhance diagnostic support. However, laboratory testing cannot be ignored for its role in excluding other diseases, and active surveillance can also assist in determining the severity and progression of persistent infections.
Diagnosis for children COVID-19 can be confirmed by Reverse transcriptase - Polymerase chain reaction (RT-PCR) on respiratory specimen (commonly nasopharyngeal and oropharyngeal swab). Severe pneumonia and critically ill children require admission and aggressive management. An early intubation is preferred over non-invasive ventilation or heated, humidified, high flow nasal cannula oxygen, as these may generate aerosols increasing the risk of infection in health care personnel. To prevent post discharge dissemination of infection, home isolation for 1–2 weeks may be advised. Children with mild disease also need to be hospitalized; if this is not feasible, these children may be managed on ambulatory basis with strict home isolation. Due to the lack of previous experience in treating children during this outbreak, some questionable decisions were made based on adult guidelines. There is currently no specific drug for patients infected with SARS-COV-2. In our study, all children were administered with interferon antiviral treatment, which was similar to the treatment recommendations for children created by The Zhejiang University School of Medicine. The proposal recommended the use of interferon atomization for children with complications [such as acute respiratory distress syndrome (ARDS), encephalitis, hemophagocytic syndrome, or septic shock], and the use of intravenous immunoglobulin in severe cases (15). However, since none of these therapies have shown any significant advantage in treating other types of coronavirus infection, it is doubtful whether they will be beneficial in treating COVID-19 infection. Even so, some studies have recommended lopinavir/ritonavir for children infected with COVID-19; our center advocated the oral administration of oseltamivir granules, which was beneficial to prognosis, and no one required glucocorticoid or immunoglobulin therapy (16). However, we now acknowledge that the novel Coronavirus infection appears to be self-limited in most children without underlying diseases, making it questionable whether antiviral drugs are needed. At the same time, the mechanism of TCM treatment merits further study, although the guidelines already currently recommend its use (17). The therapeutic focus for children infected with COVID-19 should be on supportive therapies, especially in ensuring adequate fluid and calorie intake, additional oxygen supplementation, and various avenues for maintaining the physical, mental, and emotional health of growing children. The aim should be to prevent ARDS, organ failure, and secondary nosocomial infections. If bacterial infection is suspected, broad-spectrum antibiotics such as second-generation cephalosporins or the third-generation cephalosporins can be used (18-20).
In the follow-up period of 2 months for the 7 participants, nobody presented any symptoms of discomfort. A total of 6 children were repeatedly reexamined with throat swab nucleic acid tests 2 weeks after discharge which returned negative results, and 1 child returned consistently positive in throat swab nucleic acid tests 1 month after discharge, which indicated a low re-positive rate. The child with diarrhea who was 3 month and 14 days old was negative for SARS-COV-2 according to their test on the 14th day after admission, and the child had no clinical symptoms. However, this participants’ anal swabs were returned as negative only once during his hospitalization and at 2-month follow-up after discharge, with the remaining SARS-COV-2 tests having showed positive every time. Although no nucleic acid sequence analysis was performed on this child, the possibility of repeated infection was unlikely under our strict isolation control. The only negative anal swab test may have been a false negative due to a low viral load, so the possibility of fecal-oral route of transmission of coronavirus should be assessed. Personal hygiene during family quarantine should be noted. However, further studies are needed to verify whether the positive results of pharyngeal swabs could be due to viral nucleic acid fragments or live viruses, and the clinical significance of the continuous positive results of anal swabs in children and the duration of the virus in the intestines still needs to be further investigated. The median value of immunoglobulin M (IgM) of novel Coronavirus antibody was 4.05 AU/mL (1.08–18.17 AU/mL), and the median value of immunoglobulin G (IgG) of novel Coronavirus antibody was 101.7 AU/mL (3.16–108.89 AU/mL), which indicated that the antibody had been produced and the immune response to the virus had been established: the children were already recovering and safe.
Above all, COVID-19 infections in children in Jingzhou were found to be caused by familial aggregation, were not serious, and had good prognoses. Asymptomatic children could have been infected by contact with confirmed family members. The clinical manifestation of COVID-19 infection in children are mostly non-specific, and fever and/or cough may be the only presenting symptoms. It is difficult to differentiate pediatric COVID-19 infection from other infectious diseases, and nonspecific laboratory tests may also confuse the diagnostic process, which necessitates the combination of clinical epidemiology, nucleic acid testing of novel coronavirus, and the chest CT examination, among others, to provide a scientific reliable basis for the diagnosis of infection in children. Meanwhile, attention must be given to the potential for children to be asymptomatic carriers in order to prevent and control the epidemic.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/tp-21-48
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-21-48
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-21-48). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First People’s Hospital of Jingzhou (NO.:20201006) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536-44. [Crossref] [PubMed]
- Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi 2020;58:269-74. [PubMed]
- Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr 2020;16:251-9. [Crossref] [PubMed]
- Choi SH, Kim HW, Kang JM, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr 2020;63:125-32. [Crossref] [PubMed]
- Dhochak N, Singhal T, Kabra SK, et al. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr 2020;87:537-46. [Crossref] [PubMed]
- Koyama T, Platt D, Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ 2020;98:495-504. [Crossref] [PubMed]
- Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:4. [Crossref] [PubMed]
- . Team CC-R. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422-6. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Kanne JP, Chest CT. Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020;295:16-7. [Crossref] [PubMed]
- Song F, Shi N, Shan F, et al. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020;295:210-7. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 2020;16:240-6. [Crossref] [PubMed]
- Sankar J, Dhochak N, Kabra SK, et al. COVID-19 in Children: Clinical Approach and Management. Indian J Pediatr 2020;87:433-42. [Crossref] [PubMed]
- Lin L, Yan H, Chen J, et al. Application of metabolomics in viral pneumonia treatment with traditional Chinese medicine. Chin Med 2019;14:8. [Crossref] [PubMed]
- Martinez EE, Mehta NM. The science and art of pediatric critical care nutrition. Curr Opin Crit Care 2016;22:316-24. [Crossref] [PubMed]
- Beranger A, Pierron C, de Saint Blanquat L, et al. Communication, information, and roles of parents in the pediatric intensive care unit: A review article. Arch Pediatr 2017;24:265-72. [PubMed]
- Coats H, Bourget E, Starks H, et al. Nurses' Reflections on Benefits and Challenges of Implementing Family-Centered Care in Pediatric Intensive Care Units. Am J Crit Care 2018;27:52-8. [Crossref] [PubMed]


