Case report of juvenile polyposis/hereditary hemorrhagic telangiectasia syndrome: first report in Korea with a novel mutation in the SMAD4 gene
IntroductionOther Section
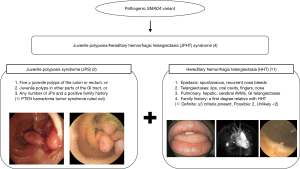
Juvenile polyposis syndrome (JPS) is a rare autosomal dominant condition, characterized by multiple hamartomatous polyps throughout the gastrointestinal (GI) tract which increases the risk of development of GI malignancy (1). Well known genetic mutations associated with JPS are the SMAD4 or BMPR1A genes which are found in 40% to 60% of patients with JPS (2). Patients with SMAD4 mutation may also have clinical features of hereditary hemorrhagic telangiectasia (HHT). HHT is a vascular malformation disorder characterized by mucocutaneous telangiectases, epistaxis, and arteriovenous malformations (AVMs) that may occur in the lungs, liver, brain, and GI tract (3). This rare overlapping syndrome of JPS and HHT caused by mutations in the SMAD4 gene is termed JPS/HHT syndrome (JPHT; MIM# 175050) (1,4-6).
Reports of JPHT syndrome with genetically confirmed SMAD4 variants have been reported worldwide mostly in Western countries (1-9). To our knowledge none has been reported in Korea. Herein, we report the first case of genetically confirmed JPHT syndrome in Korea. Moreover, we report a novel deletion variant in the SMAD4 gene that has never been reported.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tp-21-12).
Case presentationOther Section
A 15-year-old Korean boy with clinically diagnosed JPS was referred due to recurrent hematochezia. He had been followed at a tertiary hospital from 7 years of age for JPS, where he had received polypectomy annually. Thirty to 50 colonic polyps were observed at each exam, and histologic exams of the polypectomized specimens were compatible for juvenile polyps. Esophagogastroduodenoscopy and small bowel series conducted along with polypectomies were unremarkable. He had frequent events of epistaxis since 5 years of age. The patient was the only child in the family and past medical history of the parents were unremarkable.
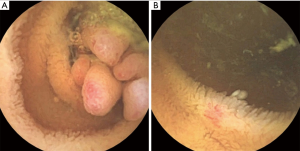
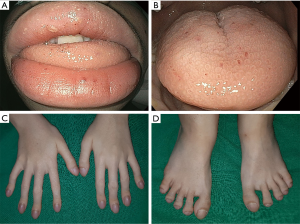
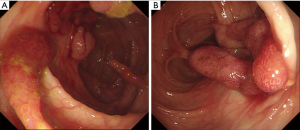
On admission, his vital signs were normal. Physical examination revealed telangiectases on the lip and tongue, and digital clubbing was observed on the extremities (Figure 1). Initial laboratory tests showed a white blood cell count 7,170/µL, hemoglobin 9.4 g/dL, hematocrit 32.1%, platelet count 432,000/µL, serum iron 13 µg/dL, ferritin <13 ng/mL, total iron-binding capacity 443 µg/dL. Other laboratory tests were all in normal range. Ileocolonoscopy showed more than 50 pedunculated and sessile polyps throughout the colon and rectum (Figure 2), and polypectomy was conducted. Histologic exams of the polypectomized specimens were compatible for juvenile polyps. Esophagogastroduodenoscopy was unremarkable.
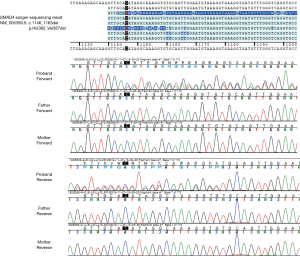
Diagnostic exome sequencing and bioinformatic analyses revealed a novel variant, SMAD4 c.1146_1163del; p.His382_Val387del (NM_005359.5). Exome sequencing data revealed no mosaicism in the patient. The variant was confirmed by Sanger sequencing on the patient. The target site of the variant and the flanking DNA sequences from the patient was amplified with forward and reverse primers. Sanger sequencing on his parents identified the c.1146_1163del as a de novo variant (Figure 3).
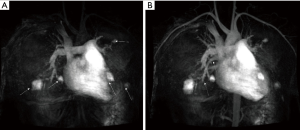
Capsule endoscopy revealed 10 small polyps and telangiectases were observed in the jejunum (Figure 4). Considering the possibility of coexisting AVMs in other organs, further evaluation was conducted. Magnetic resonance (MR) imaging and angiography of the brain was unremarkable. Computer tomographic (CT) angiography of the abdomen was also unremarkable, while MR angiography of the chest revealed a total five AVMs in both lungs with feeding vessels sizes of 3–4 mm (Figure 5). Transthoracic echocardiography was unremarkable. The patient was diagnosed with JPHT syndrome. The patient is currently scheduled for embolization of the feeding vessels.
This case report was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital (Number 2020-09-012). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
DiscussionOther Section
A common underlying genetic mutation responsible for both syndromes of JPS and HHT had been suggested after the first case report of JPS with pulmonary AVMs in 1980 (10). The genetic basis of this overlapping syndrome of JPS and HHT was first revealed by Gallione et al. in 2004 (4), reporting that mutations in one of the previously known genes involved in the pathogenesis of JPS, the SMAD4 gene, was also capable of causing features of HHT. This overlapping syndrome of JPS and HHT is now termed JPHT syndrome (Figure 6).
Clinical manifestations are variable among patients with SMAD4 variants (1). While some patients present with JPS alone, some present with features of both JPS and HHT, suggesting a poor genotype-phenotype correlation between SMAD4 and HHT features (6). Meanwhile, O’Malley et al. reported that the majority of patients with SMAD4 variants presenting with features of only JPS were also shown to have features of HHT when clinical evaluation was focused on these specific findings (8). Conversely, patients with SMAD4 variants presenting with features of only HHT were found to all have asymptomatic colonic polyps (5). Therefore, as previous studies have indicated, any JPS patient with a genetically confirmed SMAD4 variant should undergo further radiologic evaluation for asymptomatic AVMs, and any HHT patient with a genetically confirmed SMAD4 variant should undergo endoscopic evaluation for GI polyps (1,4-6).
The diagnosis of HHT is based on the Curaçao criteria: (I) spontaneous and recurrent epistaxis; (II) multiple mucocutaneous telangiectases at characteristic sites, such as the lips, oropharynx, fingers, and nose; (III) visceral involvement, including GI telangiectases, pulmonary, hepatic, brain and spinal AVMs; and (IV) a first-degree family history of HHT. The diagnosis of HHT is definite when three criteria are met, possible when two criteria are met, and unlikely when less than two criteria are met (11). The patient met the first three Curaçao criteria which corresponds to a definite diagnosis of HHT. Studies have shown that findings of HHT, such as epistaxis, mucocutaneous telangiectases, and digital clubbing due to pulmonary AVMs are observed frequently in JPHT even in early childhood, and therefore may provide a clue to the diagnosis of JPHT in children with JPS (1,8,9). Epistaxis was observed in 61–71% of patients with JPHT starting at childhood (1,8,9), while pulmonary AVMs were observed in 53–81% of patients with JPHT observed as early at birth (1,8,9). Mucocutaneous telangiectases was observed in 48% of patients with JPHT aging from 5–65 years. Similarly, in this case, frequent events of epistaxis were present since 5-year-old, and mucocutaneous telangiectases and digital clubbing was observed at presentation to our hospital. However, we were unable to suspect JPHT at first glance, and the diagnosis of JPHT was only capable after a SMAD4 variant was detected leading to further evaluation and findings of pulmonary AVMs and telangiectases in the jejunum.
To our knowledge the variant in this patient has never been reported. According to the Sequence Interpretation Guidelines of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (12), the c.1146_1163del variant could be classified as a likely pathogenic variant (LPV) based on the following evidence: it is absent in the gnomAD population database (PM2), de novo variant in a patient with the disease and no family history (PS2), patient’s phenotype or family history is highly specific for a disease with a single genetic etiology (PP4).
According to studies from Western countries, the incidence of JPS is approximately 1 in 16,000 to 100,000 and 27% of JPS is attributed to pathogenic variants in the SMAD4 gene (1). Meanwhile, there is scarce data regarding the distribution of pathogenic SMAD4 variants among the East Asian population, and few cases of JPHT have been reported in literature (13). To date this is the first case of JPHT syndrome reported in Korea. There have been reports of JPS patients with SMAD4 variants (14,15). However, no features of HHT were reported in these patients (14,15). Meanwhile, a recent multicenter case series investigating the genetic analysis of HHT in Korea reported that among 49 patients who had underwent genetic testing, 28 had ENG mutations and 19 had ACVRL1 mutations, while the other two patients were all negative for ENG, ACVRL1, and SMAD4 mutations (16). Similarly, a study in Chinese families with HHT revealed variants in ACVRL1 and ENG, while no SMAD4 mutations were detected (17).
Patients with JPS are at a high risk of developing early-onset GI cancers (1,7,18). It is well known that the loss of tumor suppressor proteins, including transforming growth factor-β (TGF-β) is crucial in the development of colorectal cancers (19). TGF-β signaling reduces proliferation and promotes apoptosis and differentiation in colon epithelial cells, and therefore loss of TGF-β signaling leads to cancer development (19). The SMAD4 protein belongs to the TGF-β family and plays a role in signaling from the cell membrane to the nucleus (19). Because of this potential of cancer development, upper GI endoscopy and colonoscopy surveillance is recommended to start from 12 to 15 years of age and repeated in intervals of 1–5 years (2).
Treatment of pulmonary AVMs is indicated when the patient has symptoms such as dyspnea, exercise intolerance, and hypoxemia (3). However, embolization should also be considered in asymptomatic patients who have pulmonary AVMs with feeding vessels of a diameter ≥3.0 mm in order to prevent paradoxical thrombotic and septic emboli and massive bleeding (20). In this patient, AVMs with feeding vessels sizes of 3–4 mm were detected on MR angiography of the chest. The patient is currently scheduled for embolization of the feeding vessels in order to prevent these complications.
In conclusion, we report the first case of JPHT in Korea, with a novel variant in the SMAD4 gene. Patients with JPS should undergo genetic evaluation of associated genes including SMAD4, and those with genetically confirmed SMAD4 variants should undergo proper evaluation for coexisting asymptomatic AVMs in order to prevent life-threatening complications.
AcknowledgmentsOther Section
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C1011004) and granted to Ben Kang.
FootnoteOther Section
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tp-21-12
Peer Review File: Available at http://dx.doi.org/10.21037/tp-21-12
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-21-12). Dr. BK reports grants from National Research Foundation of Korea (NRF) funded by the Korean government (MSIT), during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This case report was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital (Number 2020-09-012). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Larsen Haidle J, Howe JR. Juvenile Polyposis Syndrome. 2003 May 13 [updated 2017 Mar 9]. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2020. [Accessed October 15, 2020].
- Cohen S, Hyer W, Mas E, et al. Management of Juvenile Polyposis Syndrome in Children and Adolescents: A Position Paper From the ESPGHAN Polyposis Working Group. J Pediatr Gastroenterol Nutr 2019;68:453-62. [Crossref] [PubMed]
- McDonald J, Pyeritz RE. Hereditary Hemorrhagic Telangiectasia. 2000 Jun 26 [updated 2017 Feb 2]. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2020. [Accessed October 15, 2020].
- Gallione CJ, Repetto GM, Legius E, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004;363:852-9. [Crossref] [PubMed]
- Gallione CJ, Richards JA, Letteboer TG, et al. SMAD4 mutations found in unselected HHT patients. J Med Genet 2006;43:793-7. [Crossref] [PubMed]
- Gallione C, Aylsworth AS, Beis J, et al. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am J Med Genet A 2010;152A:333-9. [Crossref] [PubMed]
- Schwenter F, Ratjen F, Berk T, et al. Juvenile polyposis syndrome, SMAD4 mutations, and hereditary hemorrhagic telangiectasia. J Pediatr Gastroenterol Nutr 2012;54:120-2. [Crossref] [PubMed]
- O'Malley M, LaGuardia L, Kalady MF, et al. The prevalence of hereditary hemorrhagic telangiectasia in juvenile polyposis syndrome. Dis Colon Rectum 2012;55:886-92. [Crossref] [PubMed]
- Wain KE, Ellingson MS, McDonald J, et al. Appreciating the broad clinical features of SMAD4 mutation carriers: a multicenter chart review. Genet Med 2014;16:588-93. [Crossref] [PubMed]
- Cox KL, Frates RC Jr, Wong A, et al. Hereditary generalized juvenile polyposis associated with pulmonary arteriovenous malformation. Gastroenterology 1980;78:1566-70. [Crossref] [PubMed]
- Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000;91:66-7. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Hashimoto Y, Yokoyama K, Kumagai H, et al. Juvenile polyposis syndrome-hereditary hemorrhagic telangiectasia associated with a SMAD4 mutation in a girl. Clin J Gastroenterol 2020;13:1096-101. [Crossref] [PubMed]
- Kim IJ, Ku JL, Yoon KA, et al. Germline mutations of the dpc4 gene in Korean juvenile polyposis patients. Int J Cancer 2000;86:529-32. [Crossref] [PubMed]
- Jee MJ, Yoon SM, Kim EJ, et al. A novel germline mutation in exon 10 of the SMAD4 gene in a familial juvenile polyposis. Gut Liver 2013;7:747-51. [Crossref] [PubMed]
- Kim D, Seo EJ, Song YS, et al. Current Status of Clinical Diagnosis and Genetic Analysis of Hereditary Hemorrhagic Telangiectasia in South Korea: Multicenter Case Series and a Systematic Review. Neurointervention 2019;14:91-8. [Crossref] [PubMed]
- Zhao Y, Zhang Y, Wang X, et al. Variant analysis in Chinese families with hereditary hemorrhagic telangiectasia. Mol Genet Genomic Med 2019;7:e893 [Crossref] [PubMed]
- Aytac E, Sulu B, Heald B, et al. Genotype-defined cancer risk in juvenile polyposis syndrome. Br J Surg 2015;102:114-8. [Crossref] [PubMed]
- Jung B, Staudacher JJ, Beauchamp D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017;152:36-52. [Crossref] [PubMed]
- Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73-87. [Crossref] [PubMed]