Cesarean section and pregnancy outcomes of preterm premature rupture of membranes under different fertility policies in China
Introduction
Preterm premature rupture of membranes (PPROM), defined as the rupture of fetal membranes prior to 37 weeks of completed gestation, is a severe obstetric problem with an incidence rate of 3–4% in all pregnancies (1-3). PPROM is associated with brief latency, infectious complications, and adverse neonatal outcomes (4). As one of the most common causes for preterm delivery, premature rupture is due to either physiological weakening combined with shearing forces created by uterine contractions or various pathological factors, including an incompetent cervix, hydramnios, trauma, and amniotic fluid infection (5,6). Generally, preterm births account for 75% of perinatal mortality and lead to an increased risk of impairment in the development of the nervous system, respiratory system, and other systems (7,8). Specifically, premature infants may suffer from intraventricular hemorrhage, periventricular leukomalacia, respiratory distress syndrome, necrotizing enterocolitis, neonatal sepsis, cardiac abnormalities, and even long-term diseases (9). All of these could result in psychological and financial burdens on the family and society.
In China, with the continuous development of society and the economy, government policies and people’s view of childbirth are changing. In 2012, the government’s 1-child policy, which had been implemented for more than 3 decades, was still in existence. In 2014, this was changed to the 2-child policy in cases where either the husband or the wife was from a single-child family. In 2017, the government further relaxed family planning policy and the 2-child policy was implemented for all couples. Therefore, the 3 time points representing different guiding policies and are worthy of study in terms of outcomes of pregnancies complicated by PPROM.
In the present study, we aimed to describe and compare the incidence rate, risk factors, delivery mode of patients with PPROM, and outcomes of the infants in 2012, 2014, and 2017. We present the following article in accordance with the STROBE reporting checklist (available at
Methods
Population
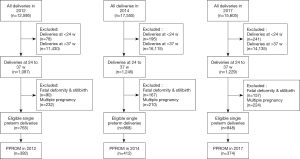
The present study was approved by the Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Beijing, China. Clinical records in our study were used with the informed consent of each participant. This study was a retrospective review of women with preterm delivery, particularly those with PPROM, who were admitted to Beijing Obstetrics and Gynecology Hospital in 2012, 2014, and 2017 (from January 1 to December 31 for each year). Deliveries at <24 and >37 weeks, fatal deformities, stillbirths, and multiple pregnancies were excluded. The selection of patients is strictly in accordance with the process shown in Figure 1. The personnel who collected and analyzed the data were not aware of the purpose of the experiment. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Diagnosis and management of PPROM
Rupture of the membranes of the amniotic sac and chorion more than 1 h prior to the onset of labor was regarded as premature rupture of membranes, and premature rupture of membranes that occurred prior to 37 weeks of completed gestation was further regarded as PPROM. The measurement of gestational weeks was determined by the date of the last menstrual period (LMP) and first trimester ultrasonography; fetal size at early pregnancy, measured by B-ultrasound, was used for women with unknown LMP or irregular menstrual periods.
In addition to symptoms and previous medical history, a sterile speculum examination was used for the diagnosis of PPROM. Symptoms of PPROM included amniotic fluid passing through the cervix and pooling of fluid in the posterior fornix. Clinical laboratory test included the measurement of pH by nitrazine paper reaction and ferning pattern, and the expression of biomarker like insulin-like growth factor binding protein-1.
For the management of PPROM, couples were counseled about the possible outcomes of both delivery and conservative management, followed by a detailed discussion regarding their choice. Patients chose conservative management when not in labor at admission. Antenatal corticosteroids (4 doses of 5 mg intramuscular betamethasone within a 48-h interval) were administrated, and fetal monitoring, including ultrasonography and non-stress testing, were performed. Maternal body temperature, maternal heart rate, serum C-reactive protein levels, and white blood cell count were monitored in case of clinical chorioamnionitis.
Data collection
Maternal parameters, including age, number of pregnancies, number of deliveries, number of cesarean section (CS), post-in vitro fertilization, weeks of gestation, CS indication, and final delivery mode were reviewed. Various obstetric complications were recorded, including pregnancy-induced hypertension (PIH), gestational diabetes mellitus, hypothyroidism, postpartum hemorrhage, malposition of fetus, placental abruption, placenta previa, intrauterine infection, prolapse of cord, uterine fibroid, cervical incompetence, cervical cerclage, vaginitis, fetal growth restriction (FGR), group B streptococcus infection, miscarriage/stillbirth, uterine septum/unicornuate uterus/bicornuate uterus, hydrops fetalis, hysterorrhexis, vasa previa rupture, prenatal amniotic fluid embolism, and puerperal infection. Fetal parameters, including fetal distress, birth weight, body length, neonatal mortality, and Apgar score at 1, 5, and 10 min, were studied.
Statistical analyses
SPSS (version 20.0; IBM, Armonk, NY, USA) was used for data analysis. Continuous variables were shown as mean ± standard deviation; χ2-test was used for the comparison of multiple ratios and composition ratios. Categorical variables were analyzed using non-parametric tests. To calculate the influencing factors, the logistic regression model was used. In addition, one-way ANOVA was used in the evaluation of body weight, body length, and Apgar score (1, 5, and 10 min). P<0.05 was considered statistically significant.
Results
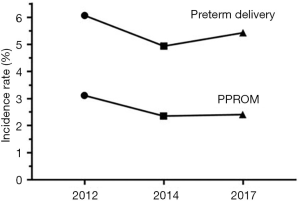
Of a total 12,595 women in 2012, 17,555 women in 2014, and 15,605 women in 2017, deliveries at <24 weeks (78 in 2012, 195 in 2014, and 241 in 2017) and at >37 weeks (11,430 in 2012, 16,115 in 2014, and 14,135 in 2017) were excluded. Fatal deformities and stillbirths (90 in 2012, 167 in 2014, and 157 in 2017), and multiple pregnancies (232 in 2012, 210 in 2014, and 224 in 2017) were further excluded. Finally, 765 (in 2012), 868 (in 2014), and 848 (in 2017) eligible single preterm deliveries remained (Figure 1). The incidence rate of preterm delivery was 6.07% for 2012, 4.94% for 2014, and 5.43% for 2017 (Figure 2). Of these, PPROM occurred at a rate of 3.11% in 2012, 2.35% in 2014, and 2.40% in 2017 (Figure 2). By comparison, the incidence rate of PPROM was significantly different among different time groups (P<0.001), and seemed to decrease in 2014 and 2017 compared with 2012 (Table 1). We further study the incidence rate of PPROM at different weeks of gestation divided into 24–27+6 weeks, 28–33+6 weeks, and 34–36+6 weeks (Table 2). At 24–27+6 weeks and 28–33+6 weeks, no significant difference was found among the 3 years. However, for pregnancies at 34–36+6 weeks, the incidence rate of PPROM was shown to decrease from 57.9% in 2012 to 53.7% in 2014 and to 49.1% in 2017 (P=0.024).

Full table

Full table
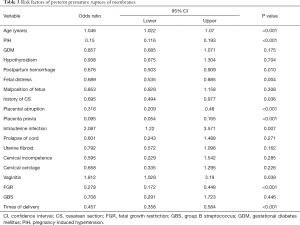
Factors influencing pregnancies complicated by PPROM were evaluated (Table 3). Age, PIH, postpartum hemorrhage, fetal distress, times of CS, placental abruption, placenta previa, intrauterine infection, vaginitis, FGR, and times of delivery were possibly influencing factors (P<0.05). Of note, age, intrauterine infection, and vaginitis were risk factors on the basis of the odds ratio (OR) value.
Full table
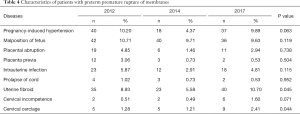
As shown in Table 4, the characteristics of patients with PPROM were analyzed. No statistically significant differences were found in PIH, malposition of fetus, placental abruption, placenta previa, intrauterine infection, prolapse of cord, cervical incompetence (all P>0.05), while uterine fibroid (P=0.045) and cervical cerclage (P=0.044) were found to be significantly different among different time groups.
Full table
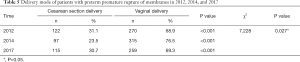
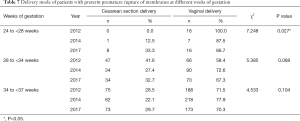
The delivery mode of patients with PPROM was also studied. In all 3 years, the findings indicated that patients with PPROM tended to choose vaginal delivery rather than CS delivery (P<0.001 for 2012, 2014, and 2017), and the rate of vaginal delivery in 2014 was higher than that in 2012 and 2017 (P=0.027) (Table 5). As shown in Table 6, the difference among different weeks of gestation in 2014 (P=0.390) and in 2017 (P=0.822) was not statistically significant. The comparison of different weeks of gestation in 2012 showed a significant difference (P=0.001), with the rate of vaginal delivery at 24–27+6 weeks as high as 100%, and the rate of vaginal delivery at 28–33+6 weeks as low as 58.4%. As shown in Table 7, only delivery mode at 24–27+6 weeks presented a significant difference (P=0.027), and the rate of vaginal delivery demonstrated a progressive decrease from 100% (in 2012) to 87.5% (in 2014) and 66.7% (in 2017). The rate of vaginal delivery at 34–36+6 weeks was quite close in the three time groups.
Full table

Full table
Full table
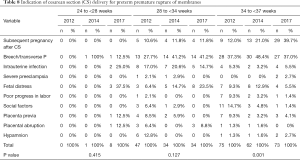
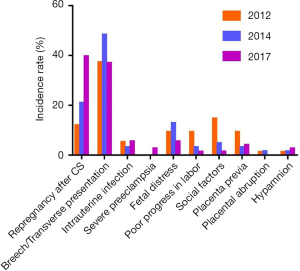
Indications of CS delivery for pregnancies complicated by PPROM are listed in Table 8. The following factors were involved: subsequent pregnancy after CS, breech/transverse presentation, intrauterine infection, severe preeclampsia, fetal distress, poor progress in labor, social factors, placenta previa, placental abruption, and hypamnion. From the data, it could be observed that breech/transverse presentation was the most common factor for PPROM at 28–33+6 weeks. The results also showed that indications at 34–36+6 weeks varied significantly among the 3 years (P=0.001). As shown in Figure 3, the incidence rate of subsequent pregnancy after CS and hypamnion increased progressively, and the incidence rate of poor progress in labor and social factors decreased progressively. Notably, the incidence rate of subsequent pregnancy after CS at 34–36+6 weeks increased significantly, and the incidence rate of intrauterine infection at 34–36+6 weeks decreased significantly, compared with 28–33+6 weeks.
Full table
Body weight, body length, and Apgar score after 1, 5, and 10 min for neonates following PPROM were recorded and compared. A trend of decrease in body weight at 28–33+6 weeks was observed; however, there was no significant difference (P=0.069). Body length showed a progressive decrease from 2012, 2014, to 2017 (P=0.037). For Apgar score, only 1-min evaluation for neonates at 28–33+6 weeks was significantly different among different time groups (Table 9).
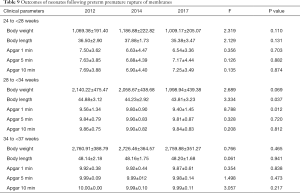
Full table
Discussion
The present single-center study was based on a large population of 45,755 women; a total of 1,178 pregnancies complicated by PPROM totally were analyzed. The incidence rate of PPROM at our single center varied between 2012, 2014, and 2017. Risk factors for pregnancies complicated by PPROM include age, intrauterine infection, and vaginitis. The rate of CS delivery varied, and breech/transverse presentation was the major indication for patients with PPROM at 34–36+6 weeks.
The incidence rate of PPROM was around 3% in 2012 and a decreasing trend was observed in both 2014 and 2017. In the same period, nearly one-third of preterm births were complicated by PPROM in the USA (10). Our findings indicated that PPROM complicated approximately half of preterm deliveries in a single-center study (Beijing, China), which encouraged us to further explore the cause. With time, the incidence of PPROM seems to decrease, particularly at 34–36+6 weeks. The Chinese government's fertility policy and public opinion regarding reproduction are changing gradually. It is very important to improve the management of PPROM from the accumulated experience. Tocolytic agents could help prolong the latency period of impending labor, which allows for the full effects of lung maturation, following the administration of corticosteroids (11,12). Meanwhile, clinicians need a way to balance the benefits of prolonged pregnancy with the risk of maternal and infant amniotic infection (13,14). It has been reported that antibiotics could reduce complications due to preterm delivery and post-natal infection in high-income areas (13).
Based on the OR values, age, intrauterine infection, and vaginitis were possible risk factors for PPROM. There are many risk factors for premature rupture of fetal membranes, most of which are related to infection. Multiple mechanisms of the PPROM have been reported in the literature, including pathologic anatomical remodeling, invasive procedures, fetoscopic surgeries, genetic and iatrogenic factors, and inflammation (11). The supracervical area is the most prevalent site for PPROM, where the amniotic membrane is structurally altered, easily disrupted, and often laden with bacteria (15). Inflammation, especially chorioamnionitis, plays an important role in the occurrence of PPROM (9,11). Modi et al. found that mutations in fetal genes involved in innate immunity and host defense against microbes could increase the risk of PPROM in African American mothers, suggesting that microbial infection and microbial products may be harmful factors may be the hazard (16). Oh et al. have found that the earlier the gestational age at the time of PPROM, the higher the intensity of the intra-amniotic inflammatory response in women with preterm PPROM (17). Sae-Lin et al. reported that diabetes mellitus, insufficient maternal weight gain, and history of previous preterm births significantly increased the risk of PPROM (with a 5-year incidence of 2.93%) (18). Our study found that intrauterine infection and vaginitis are risk factors, which may be involved in inflammation leading to PPROM. Furthermore, given that medical technology has been improving in China, less inflammation might be a factor accounting for the decrease in the incidence rate of PPROM, as previously mentioned. Studies have reported that infection may be the initial cause of PPROM (5,19). However, there is no consensus on whether infection is a cause or result (1). Lorthe et al. Identified many risk factors, but found few that could be changed (20). These findings are also consistent with the reports of Merello et al. (21).
Delivery mode is another important risk factor of PPROM. The percentage of CS deliveries has been decreasing in the USA, which is in contrast to 1 decade ago when the percentage of CS was rising in almost all high-income countries (22,23). At our center (Beijing, China), more patients with PPROM chose vaginal delivery in 2012, 2014, and 2017. The rate of CS delivery in 2014 (when the 2-child policy initially began) was ~7% lower than that in 2012 (when the 1-child policy was still in effect) and 2017 (when the 2-child policy was implemented for all couples). A cross sectional study of delivery mode involving 39 hospitals in 14 provinces of China in 2011 reported the rate of CS delivery was 54.6%, which was higher than that in our study (24). CS delivery is often requested in China (25,26). We suspect that the change of incidence rate of PPROM may also be related to the choice of delivery mode. Besides, indication of CS delivery could directly influence the choice of delivery mode for patients with PPROM. The rate of CS delivery was reported to decline with increased gestation in an international comparison of gestational age patterns in CS delivery (27). It is imperative to fully evaluate the maternal and fetal indications, which vary by weeks of gestation (28). Although the rate of CS delivery at 24–27+6 weeks increased from 2012, 2014, to 2017, the number at each year was significantly lower than that at 28–33+6 weeks and 34–36+6 weeks, making the result less reliable. Consistent with the results of Gao et al. that previous CS delivery, fetal distress, and malpresentation account for more than 50% of all CS deliveries (29), we found that breech/transverse presentation was a significant indication for patients with PPROM at 34–36+6 weeks. To date, the optimal mode of delivery is not clear (30). Racusin et al. compared the neonatal outcomes according to CS delivery and vaginal delivery, and found no evidence of improvement by CS delivery (28). On the basis of body weight, body length, and Apgar score (1, 5, and 10 min), most outcomes of neonates showed no difference, and only a few neonates may have significant differences.
In the present study, under different government fertility policies, no significant difference was found in neonatal outcomes after comparing neonatal weight and neonatal Apgar score between 5 and 10 min. These results are closely related to the principles of treatment we follow. For PPROM without other complications <34 weeks, preterm birth is the greatest risk, and seriously affects the outcome of pregnancy. Therefore, expectant treatment is often given. Infection can be prevented and fetal lung maturity treatment can be given at the same time as contraction suppression. The use of drugs to prevent pregnancy can prolong the incubation period of PPROM so that there is sufficient time to apply glucocorticoids to promote fetal lung maturity (30). At the same time, during the extension of pregnancy, we need to balance the benefits of prolonged pregnancy and the risk of intrauterine infection. For patients with PPROM at 34–37+6 weeks, who have no contraindications to pregnancy, after careful monitoring and anticipation of treatment, a better mother–infant outcome may be obtained.
Limitations
The involvement of multiple centers and a larger population in future studies would improve the reliability of our findings. As a retrospective study, the conclusions of this study still need prospective cohort study to verify the risk factors and outcomes of two delivery modes in PPROM, so as to provide meaningful guidance at a higher level of evidence.
Conclusions
The incidence rate of PPROM at our single center varied from 2012, 2014, to 2017. Risk factors for pregnancies complicated by PPROM include age, intrauterine infection, and vaginitis. The rate of CS delivery varied, and breech/transverse presentation was the major indication for patients with PPROM at 34–36+6 weeks.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-21-144
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-21-144
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-21-144). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Beijing, China. Clinical records in our study were used with the informed consent of each participant.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Menon R, Richardson LS. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin Perinatol 2017;41:409-19. [Crossref] [PubMed]
- Kibel M, Asztalos E, Barrett J, et al. Outcomes of Pregnancies Complicated by Preterm Premature Rupture of Membranes Between 20 and 24 Weeks of Gestation. Obstet Gynecol 2016;128:313-20. [Crossref] [PubMed]
- Wong LF, Holmgren CM, Silver RM, et al. Outcomes of expectantly managed pregnancies with multiple gestations and preterm premature rupture of membranes prior to 26 weeks. Am J Obstet Gynecol 2015;212:215.e1-9. [Crossref] [PubMed]
- Mercer BM, Crouse DT, Goldenberg RL, et al. The antibiotic treatment of PPROM study: systemic maternal and fetal markers and perinatal outcomes. Am J Obstet Gynecol 2012;206:145.e1-9. [Crossref] [PubMed]
- Naeye RL, Peters EC. Causes and consequences of premature rupture of fetal membranes. Lancet 1980;1:192-4. [Crossref] [PubMed]
- Practice Bulletin No. 172: Premature Rupture of Membranes. Obstet Gynecol 2016;128:e165-77. [Crossref] [PubMed]
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75-84. [Crossref] [PubMed]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261-9. [Crossref] [PubMed]
- Yu H, Wang X, Gao H, et al. Perinatal outcomes of pregnancies complicated by preterm premature rupture of the membranes before 34 weeks of gestation in a tertiary center in China: A retrospective review. Biosci Trends 2015;9:35-41. [Crossref] [PubMed]
- Clark EA, Varner M. Impact of preterm PROM and its complications on long-term infant outcomes. Clin Obstet Gynecol 2011;54:358-69. [Crossref] [PubMed]
- Tchirikov M, Schlabritz-Loutsevitch N, Maher J, et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J Perinat Med 2018;46:465-88. [Crossref] [PubMed]
- Iams JD, Romero R, Culhane JF, et al. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008;371:164-75. [Crossref] [PubMed]
- Cousens S, Blencowe H, Gravett M, et al. Antibiotics for pre-term pre-labour rupture of membranes: prevention of neonatal deaths due to complications of pre-term birth and infection. Int J Epidemiol 2010;39:i134-43. [Crossref] [PubMed]
- Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394-409. [Crossref] [PubMed]
- Akolekar R, Beta J, Picciarelli G, et al. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2015;45:16-26. [Crossref] [PubMed]
- Modi BP, Teves ME, Pearson LN, et al. Mutations in fetal genes involved in innate immunity and host defense against microbes increase risk of preterm premature rupture of membranes (PPROM). Mol Genet Genomic Med 2017;5:720-9. [Crossref] [PubMed]
- Oh KJ, Romero R, Park JY, et al. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J Perinat Med 2019;47:516-27. [Crossref] [PubMed]
- Sae-Lin P, Wanitpongpan P. Incidence and risk factors of preterm premature rupture of membranes in singleton pregnancies at Siriraj Hospital. J Obstet Gynaecol Res 2019;45:573-7. [PubMed]
- Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J Obstet Gynecol 1991;164:467-71. [Crossref] [PubMed]
- Lorthe E. Epidemiology, risk factors and child prognosis: CNGOF Preterm Premature Rupture of Membranes Guidelines. Gynecol Obstet Fertil Senol 2018;46:1004-21. [PubMed]
- Merello M, Lotte L, Gonfrier S, et al. Enterobacteria vaginal colonization among patients with preterm premature rupture of membranes from 24 to 34 weeks of gestation and neonatal infection risk. J Gynecol Obstet Hum Reprod 2019;48:187-91. [Crossref] [PubMed]
- Clapp MA, Barth WH. The Future of Cesarean Delivery Rates in the United States. Clin Obstet Gynecol 2017;60:829-39. [Crossref] [PubMed]
- Declercq E, Young R, Cabral H, et al. Is a rising cesarean delivery rate inevitable? Trends in industrialized countries, 1987 to 2007. Birth 2011;38:99-104. [Crossref] [PubMed]
- Hou L, Hellerstein S, Vitonis A, et al. Cross sectional study of mode of delivery and maternal and perinatal outcomes in mainland China. PLoS One 2017;12:e0171779 [Crossref] [PubMed]
- Wang X, Hellerstein S, Hou L, et al. Caesarean deliveries in China. BMC Pregnancy Childbirth 2017;17:54. [Crossref] [PubMed]
- Zhang J, Liu Y, Meikle S, et al. Cesarean delivery on maternal request in southeast China. Obstet Gynecol 2008;111:1077-82. [Crossref] [PubMed]
- Delnord M, Blondel B, Drewniak N, et al. Varying gestational age patterns in cesarean delivery: an international comparison. BMC Pregnancy Childbirth 2014;14:321. [Crossref] [PubMed]
- Racusin DA, Antony KM, Haase J, et al. Mode of Delivery in Premature Neonates: Does It Matter? AJP Rep 2016;6:e251-9. [Crossref] [PubMed]
- Gao Y, Xue Q, Chen G, et al. An analysis of the indications for cesarean section in a teaching hospital in China. Eur J Obstet Gynecol Reprod Biol 2013;170:414-8. [Crossref] [PubMed]
- Bond DM, Middleton P, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome. Cochrane Database Syst Rev 2017;3:CD004735 [Crossref] [PubMed]
(English Language Editor: R. Scott)