
Urinary proteome profiling for children with autism using data-independent acquisition proteomics
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by difficulties in social interaction and limited repetitive behaviors, interests, or activities (1). The incidence of autism has continued to increase over the past two decades, with the number of patients with autism as high as 1% to 2.5% of the total population and a male/female ratio of 4:1 (2). Autism usually occurs at an early stage and is a lifelong developmental disorder that places a heavy burden on families and public health.
The etiology and pathological mechanism of autism are uncertain, which brings challenges to its diagnosis and intervention. There is currently no effective treatment for ASD, but some studies have found that behavioral interventions for autistic children can effectively alleviate their symptoms at the early stage (3). Therefore, the early diagnosis is crucial for autism. The clinical diagnosis of autism mainly relies on behavioral and cognitive assessment according to the criteria in the diagnostic and statistical manual of mental disorders, which is certain subjective. Hence, the objective and reliable biomarkers are needed for the diagnosis of autism.
Previous proteomic studies on biomarkers and pathogenetic mechanisms of ASD have focused on blood, saliva and brain tissues (4-8). However, only a few studies have used urine. Urine is a sensitive source for diseases biomarkers. Without the control of homeostatic mechanisms, urine can accumulate early changes of the whole body (9). In addition, urine collection is simple and non-invasive. There are several clinical studies showed that urine could reflect pathological changes of various diseases involving brain and nervous system, such as Alzheimer’s disease (10), familial Parkinson’s disease (11), pediatric medulloblastoma (12), and gliomas (13). However, for neuropsychiatric disorders with abnormal social behaviors such as ASD, it is unknown whether urine can show differences.
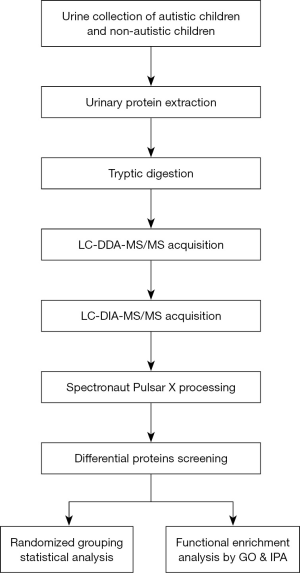
In this study, the data-independent acquisition (DIA) strategy was used to identify differential proteins in the urinary proteome between autistic and non-autistic children aged 3–7 years. This study aims to investigate whether the urinary proteome can distinguish between autistic children and non-autistic children. The workflow of this study is presented in Figure 1.
We present the following article in accordance with the MDAR reporting checklist (available at https://dx.doi.org/10.21037/tp-21-193).
Methods
Urine sample collection
In this study, urine samples from 18 autistic children aged 3–7 years from the Fengtai District Sunshine Angel Special Training Center in Beijing and 6 non-autistic children aged 3–6 years from Beijing Normal University were collected (Table S1). All ASD patients were diagnosed by child neuropsychiatrists according to criteria defined in the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-V). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) for research on human participants, and the study protocols were approved by the Institutional Review Board at Beijing Normal University (ICBIR_A_0098_006). Written informed consent was obtained from the parents of all participants.
Urinary protein extraction and tryptic digestion
Urine samples were centrifuged at 12,000 ×g for 40 min at 4 °C to remove impurities and large cell debris. The supernatants were precipitated with three volumes of ethanol at −20 °C overnight and then centrifuged at 12,000 ×g for 30 min at 4 °C. The precipitate was resuspended in lysis buffer [8 mol/L urea, 2 mol/L thiourea, 50 mmol/L Tris, and 25 mmol/L dithiothreitol (DTT)]. The Bradford assay was used to measure the protein concentration of each sample.
The urinary proteins were digested using the filter-aided sample preparation (FASP) method (14). A total of 100 µg protein of each sample was loaded onto a 10 kDa filter device (Pall, Port Washington, NY, USA) and washed twice with UA (8 mol/L urea, 0.1 mol/L Tris-HCl, pH 8.5) and 25 mmol/L NH4HCO3. The samples were reduced with 20 mmol/L DTT (Sigma, St. Louis, USA) at 37 °C for 1 h and then alkylated with 50 mmol/L iodoacetamide (IAA, Sigma, St. Louis, USA) in the dark for 40 min. After washing once with UA and twice with 25 mmol/L NH4HCO3, the proteins were digested with trypsin (enzyme-to-protein ratio of 1:50) at 37 °C overnight. The peptide mixtures were desalted using Oasis HLB cartridges (Waters, Milford, MA, USA) and then dried by vacuum evaporation.
High-pH reversed-phased peptide fractionation
The peptide samples were dissolved in 0.1% formic acid and diluted to 0.5 µg/µL. For the generation of spectral library, 96 µg of pooled peptides from 4 µg of each sample was fractionated using a high-pH reversed-phased peptide fractionation kit (catalog number: 84868, Thermo, USA). According to the manufacturer’s instructions, 10 fractionated samples were obtained and were dried by vacuum evaporation. Then, 10 fractionated samples were dissolved in 20 µL of 0.1% formic acid. One microgram of each fraction was loaded for liquid chromatography couple with tandem mass spectrometry (LC-MS/MS) analysis in data-dependent acquisition (DDA) mode.
LC-MS/MS analysis
An EASY-nLC 1200 chromatography system (Thermo Fisher Scientific, Waltham, MA, USA) and an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) were used for mass spectrometry acquisition and analysis. The iRT reagent (Biognosys, Switzerland) was spiked at a concentration of 1:10 v/v into all urinary samples for calibration of the retention time of the extracted peptide peaks. All peptide samples were loaded on a trap column (75 µm × 2 cm, 3 µm, C18,100 Å) and a reverse-phase analysis column (75 µm × 25 cm, 2 µm, C18, 100 Å). The eluted gradient was 4–35% buffer B (0.1% formic acid in 80% acetonitrile) at a flow rate of 300 nL/min for 90 min.
In DDA mode, the parameters were set as follows: the full scan from 350 to 1,500 m/z with resolution at 120,000 and MS/MS scan with resolution at 30,000 in Orbitrap; the 30% higher-energy collisional dissociation (HCD) energy; the maximum injection time of 45 ms.
In DIA mode, 1 µg of each sample was analyzed with twice replicates. The DIA method with 36 variable windows was set for DIA acquisition (Table S2). The parameters were set as follows: the full scan from 350 to 1,500 m/z with resolution at 60,000; the DIA scan from 200 to 2,000 m/z with resolution of 30,000; the 32% HCD energy; and the maximum injection time of 100 ms. A quality control (QC) sample of the mixture from each sample was analyzed in DIA acquisition after every four samples.
Spectral library generation and data analysis
The DDA data of 10 fractions were processed using Proteome Discoverer software (version 2.1, Thermo Scientific) and searched against the Swiss-Prot Human database (released in 2018, including 20,346 sequences) appended with the iRT peptide sequence. The search parameters were set as follows: two missed trypsin cleavage sites were allowed; the parent ion mass tolerances were set to 10 ppm; the fragment ion mass tolerances were set to 0.02 Da; the carbamidomethyl of cysteine was set as a fixed modification; and the oxidation of methionine was set as a variable modification. The false discovery rate (FDR) of proteins was less than 1%. A total of 2,184 protein groups, 11,518 peptide groups and 59,341 peptide spectrum matches were identified. The search result was used to set the variable windows for DIA mode. For the generation of spectral library, the DDA raw files were imported to Spectronaut Pulsar X software (Biognosys, Switzerland). All DIA raw files were processed using Spectronaut Plusar X software with default setting. All results were filtered by a Q value cutoff of 0.01. The protein identification was based on two unique peptides.
Statistical analysis
A comparison of proteins between autistic and non-autistic group was conducted using independent samples t-test. Group differences resulting in P<0.05 were considered statistically significant. Differential proteins were screened with the following criteria: fold change in increasing group ≥1.5 and in decreasing group ≤0.67, P<0.01. Receiver operating characteristic (ROC) analysis were performed for individual proteins and protein combinations using Metaboanalyst software (https://www.metaboanalyst.ca).
Functional enrichment analysis
Functional annotation of differential proteins was performed using DAVID 6.8 (https://david.ncifcrf.gov) (15) and ingenuity pathway analysis (IPA) software (Ingenuity Systems, Mountain View, CA, USA), including biological process, cellular component, molecular function and pathways. The threshold of significance was set at a P<0.05.
Results
Identification of differential proteins in ASD urinary proteome
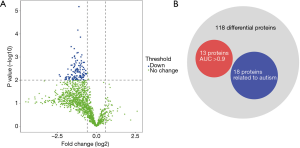
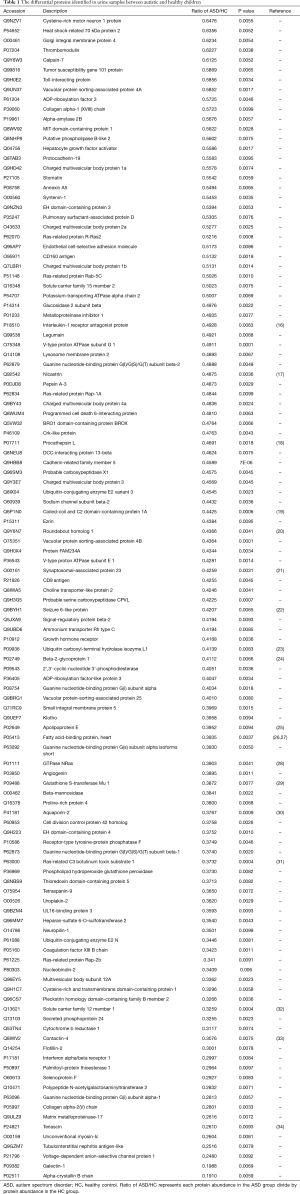
To investigate differences between autistic and non-autistic children, 24 urinary samples from 18 autistic children and 6 non-autistic children were analyzed after proteolysis by LC-DIA-MS/MS. A total of 1,631 protein groups were identified in this study. A QC sample of the mixture from each sample was analyzed after every four samples. The 95% of the quantile of coefficient of variation (CV) value of the QC was 0.51, and proteins with CV >0.51 were considered outliers. A total of 1,511 proteins with the CV below 0.51 were for subsequent analysis, and the identification and quantification details are listed in online table (available at https://cdn.amegroups.cn/static/public/tp-21-193-1.pdf). Among them, 118 differential proteins were identified between the autistic and non-autistic groups (fold change ≥1.5 or ≤0.67, P<0.01), the volcano plot of differential proteins is shown in Figure 2A. The details of the differential proteins are listed in Table 1.
Full table
Randomized grouping statistical analysis
Given that the number of proteomic features identified in the samples was higher than the number of samples, the differences between two groups might be randomly generated. A randomized grouping statistical analysis strategy was developed to confirm whether these differential proteins were caused by disease. Twenty-four samples from the autism (n=18) and control groups (n=6) were randomly divided into two groups and the same criteria were used to screen differential proteins. In a total of 134,596 () combinations, the average number of differential proteins was 10. These results showed that only 10 differential proteins could be generated randomly, further indicating that 91.5% of the differential proteins were reliable.
ROC curve analysis
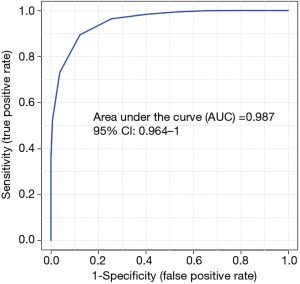
To evaluate the diagnostic performance of differential proteins between autistic and non-autistic children, ROC curves were performed for individual proteins and protein combinations. Among 118 differential proteins, 13 proteins (CDHR5, VPS4B, NICA, LEG1, ARL3, MANBA, VATG1, CO5A2, CHM1B, CDC42, NRP1, F13B, INAR1) showed the good discriminative performance between autistic and non-autistic children (AUC >0.9) (Figure 2B, Table 2). As shown in Figure 3, the combination of CDHR5 and VPS4B showed an AUC of 0.987, which was higher than that of the individual protein. Thus, these differential proteins and protein panels could be potential diagnostic biomarkers for autism.

Full table
Function analysis of the differential proteins
Functional annotation of 118 differential proteins was performed by DAVID. The differential proteins were classified into biological process, cellular component, and molecular function. In the biological process category, 62 items were significantly enriched (Table S3), of which representative biological processes are presented in Figure 4A. These differential proteins were involved in viral budding via host endosomal sorting complex required for transport (ESCRT) complex, multivesicular body assembly, autophagy, small GTPase mediated signal transduction, Ras protein signal transduction, axon guidance, chemical synaptic transmission, and negative regulation of neuron death. In the cellular component category, the majority of differential proteins came from extracellular exosomes (Figure 4B). In the molecular function category, GTPase activity, GTP binding, signal transducer activity and protein homodimerization activity were overrepresented (Figure 4C).
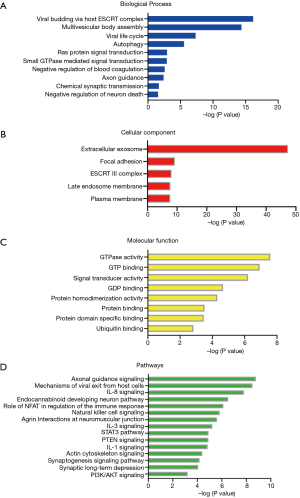
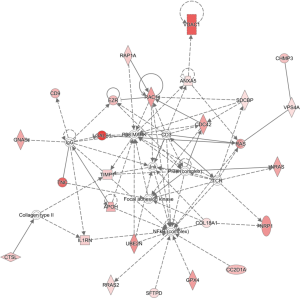
To identify the major biological pathways of differential proteins, IPA software was performed for canonical pathways and network analysis. A total of 206 items were significantly enriched (online table available at https://cdn.amegroups.cn/static/public/tp-21-193-2.pdf), of which representative pathways are presented in Figure 4D. Axonal guidance signaling, endocannabinoid developing neuron pathway, STAT3 pathway, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling, synaptogenesis signaling pathway, synaptic long-term depression, and PI3K/AKT signaling were overrepresented. In addition, IPA network analysis revealed that a total of 25 differential proteins were involved in the top regulator effect network “cell-to-cell signaling and interaction, cellular movement, hematological system development and function” with score 47 (Figure 5).
Discussion
In this study, urine proteome in children with autism was analyzed by DIA proteomics, and 118 differential proteins were identified between autistic and non-autistic children. Among them, 18 proteins have been reported to be related to autism (Figure 2B). For example, interleukin 1 receptor antagonist protein (IL1RA) is an anti-inflammatory cytokine that was downregulated in the serum of autistic patients (16). Nicastrin (NCSTN) plays an important role in the regulation of short-term and long-term synaptic plasticity (17). Cathepsin L1 (CATL1) stimulates neuronal axon growth (18). CC2D1A (19) has been reported to be as candidate genes for autism. The abnormality of ROBO may cause autism by interfering with the serotonergic system or interfering with neurodevelopment (20). SNAP25 was reported to be involved in autism, seizures, and intellectual disability (21), and SNAP23 was downregulated in this study. SEZ6L (22) is a candidate gene for autism. Low levels of ubiquitin carboxy-terminal hydrolase isoenzyme L1 (UCHL1) is associated with ubiquitination interference in autism (23). Beta-2-glycoprotein 1 (APOH) was reported to be elevated in the plasma of patients with autism compared with that of control subjects (24). APOE hypermethylation is associated with ASD in the Chinese population (25). The abnormal expression of FABP7 and FABP5 genes in individuals with autism was found, and FABP3 was downregulated in the urine of ASD patients, which plays a key role in cognition and emotional behavior (26,27). NRAS (28) is a candidate gene of ASD. GSTM1 genotype may serve as a moderator of the effect of some prenatal factors on the risk of ASD (29). The expression of AQP4 in the brains of autistic patients was reported to be decreased (30). We found that AQP2 were downregulated in the urine of ASD patients. RAC1 stimulates the initiation and elongation of dendrites, Rac1/PAK/LIMK signaling promotes actin filament assembly, and actin dysregulation is a pathophysiological mechanism of autism (31). Bumetanide administration can improve the symptoms of autism (32). We found that bumetanide-sensitive sodium-(potassium)-chloride cotransporter 2 (SLC12A1) was downregulated in urine. CNTN4 plays an important role in the formation, maintenance, and plasticity of neuronal networks and disruption of contactin 4 has been reported in ASD patients (33). The mutations in the tenascin C (TNC) gene could cause sensory impairment in ASD (34). Although some differential proteins have not been reported to be related to autism, they also might serve as candidate urinary biomarkers for autism.
In addition, some important pathways were associated with autism. For example, changes in axonal microstructure are considered to be the basis of the cognitive performance of people with autism (35), several differential proteins were involved in axonal guidance signaling. Moreover, the endogenous cannabinoid system is involved in regulating many cellular functions and molecular pathways in autism, such as unbalanced glutamate and gamma-aminobutyric acid (GABA) and glutamate energy transmission, and disorders of the endogenous cannabinoid system may play an important role in the pathophysiology of autism (36,37). Dysfunction of PTEN signaling may also be combined with changes in other autism-related genes or pathways to influence social behavior (38). Multiple susceptibility genes of autism encode synaptic-related proteins and affect the formation, elimination, transmission and plasticity of synapses (39), 9 proteins (APOE, CDC42, CRKL, NRAS, RAB5C, RAC1, RAP1A, RAP2B, RRAS2) were involved in synaptogenesis signaling pathway and 7 proteins (GNAI1, GNAI3, GNAS, NRAS, RAP1A, RAP2B, RRAS2) were involved in synaptic long-term depression. A large amount of evidence suggests that inflammation may be involved in the pathophysiological process of autism, manifested as a change in proinflammatory cytokine signals (40) and several inflammation-related signals were enriched in this study, such as IL-8 and IL-3 signaling. Thus, urinary proteins might reflect the pathophysiological process of autism and provide new targets for the intervention for autism.
Although autism is a heterogeneous neurological developmental disorder with multiple etiologies, subtypes and developmental trajectories, the urinary proteome between autistic group and non-autistic group showed clear differences, suggesting that autism might have a limited number of common biological pathways (41) or the ASD patients who contributed urine samples in this study might happen to be of similar subtypes.
This preliminary study has some limitations worth noting. First, the number of participants enrolled was limited. Secondly, the subtypes of children with autism in this study was not clear, and different subtypes may have different biomarkers, so whether our findings may be extended to other subtypes of autism is uncertain. Furthermore, whether these candidate urinary biomarkers can be applicable to earlier-age autistic children is unknown. Therefore, a large number of ASD patients with earlier ages and multiple subtypes from multicenter should be considered in future studies. Despite limitations of the study, our results demonstrate that ASD can be reflected in the urine, suggesting that urine proteome is a promising approach for diagnosis of ASD.
Conclusions
The urinary proteome could distinguish between autistic children and non-autistic children. This study will provide a promising approach for future biomarker research of neuropsychiatric disorders.
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program of China (2018YFC0910202, 2016YFC1306300); the Fundamental Research Funds for the Central Universities (2020KJZX002); the Beijing Natural Science Foundation (7172076); the Beijing Cooperative Construction Project (110651103); the Beijing Normal University (11100704); and the Peking Union Medical College Hospital (2016-2.27). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-193
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tp-21-193
Peer Review File: Available at https://dx.doi.org/10.21037/tp-21-193
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-193). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) for research on human participants, and the study protocols were approved by the Institutional Review Board at Beijing Normal University (ICBIR_A_0098_006). Written informed consent was obtained from the parents of all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014;383:896-910. [Crossref] [PubMed]
- Lyall K, Croen L, Daniels J, et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health 2017;38:81-102. [Crossref] [PubMed]
- Fernell E, Eriksson MA, Gillberg C. Early diagnosis of autism and impact on prognosis: a narrative review. Clin Epidemiol 2013;5:33-43. [Crossref] [PubMed]
- Shen L, Zhang K, Feng C, et al. iTRAQ-Based Proteomic Analysis Reveals Protein Profile in Plasma from Children with Autism. Proteomics Clin Appl 2018;12:e1700085 [Crossref] [PubMed]
- Corbett BA, Kantor AB, Schulman H, et al. A proteomic study of serum from children with autism showing differential expression of apolipoproteins and complement proteins. Mol Psychiatry 2007;12:292-306. [Crossref] [PubMed]
- Ngounou Wetie AG, Wormwood KL, Russell S, et al. A Pilot Proteomic Analysis of Salivary Biomarkers in Autism Spectrum Disorder. Autism Res 2015;8:338-50. [Crossref] [PubMed]
- Amal H, Barak B, Bhat V, et al. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol Psychiatry 2020;25:1835-48. [Crossref] [PubMed]
- Abraham J, Szoko N, Natowicz MR. Proteomic Investigations of Autism Spectrum Disorder: Past Findings, Current Challenges, and Future Prospects. Adv Exp Med Biol 2019;1118:235-52. [Crossref] [PubMed]
- Gao Y. Urine-an untapped goldmine for biomarker discovery? Sci China Life Sci 2013;56:1145-6. [Crossref] [PubMed]
- Watanabe Y, Hirao Y, Kasuga K, et al. Molecular Network Analysis of the Urinary Proteome of Alzheimer's Disease Patients. Dement Geriatr Cogn Dis Extra 2019;9:53-65. [Crossref] [PubMed]
- Virreira Winter S, Karayel O, Strauss MT, et al. Urinary proteome profiling for stratifying patients with familial Parkinson's disease. EMBO Mol Med 2021;13:e13257 [Crossref] [PubMed]
- Hao X, Guo Z, Sun H, et al. Urinary protein biomarkers for pediatric medulloblastoma. J Proteomics 2020;225:103832 [Crossref] [PubMed]
- Wu J, Zhang J, Wei J, et al. Urinary biomarker discovery in gliomas using mass spectrometry-based clinical proteomics. Chin Neurosurg J 2020;6:11. [Crossref] [PubMed]
- Wiśniewski JR, Zougman A, Nagaraj N, et al. Universal sample preparation method for proteome analysis. Nat Methods 2009;6:359-62. [Crossref] [PubMed]
- Huang da W. Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44-57. [Crossref] [PubMed]
- Saghazadeh A, Ataeinia B, Keynejad K, et al. Anti-inflammatory cytokines in autism spectrum disorders: A systematic review and meta-analysis. Cytokine 2019;123:154740 [Crossref] [PubMed]
- Lee SH, Sharma M, Südhof TC, et al. Synaptic function of nicastrin in hippocampal neurons. Proc Natl Acad Sci U S A 2014;111:8973-8. [Crossref] [PubMed]
- Tohda C, Tohda M. Extracellular cathepsin L stimulates axonal growth in neurons. BMC Res Notes 2017;10:613. [Crossref] [PubMed]
- Sener EF, Cıkılı Uytun M, Korkmaz Bayramov K, et al. The roles of CC2D1A and HTR1A gene expressions in autism spectrum disorders. Metab Brain Dis 2016;31:613-9. [Crossref] [PubMed]
- Anitha A, Nakamura K, Yamada K, et al. Genetic analyses of roundabout (ROBO) axon guidance receptors in autism. Am J Med Genet B Neuropsychiatr Genet 2008;147B:1019-27. [Crossref] [PubMed]
- Najera K, Fagan BM, Thompson PM. SNAP-25 in Major Psychiatric Disorders A Review. Neuroscience 2019;420:79-85. [PubMed]
- Chapman NH, Nato AQ Jr, Bernier R, et al. Whole exome sequencing in extended families with autism spectrum disorder implicates four candidate genes. Hum Genet 2015;134:1055-68. [Crossref] [PubMed]
- Çetin İ, Tezdiğ İ, Tarakçioğlu MC, et al. Do Low Serum UCH-L1 and TDP-43 Levels Indicate Disturbed Ubiquitin-Proteosome System in Autism Spectrum Disorder? Noro Psikiyatr Ars 2017;54:267-71. [Crossref] [PubMed]
- Careaga M, Hansen RL, Hertz-Piccotto I, et al. Increased anti-phospholipid antibodies in autism spectrum disorders. Mediators Inflamm 2013;2013:935608 [Crossref] [PubMed]
- Hu Z, Yang Y, Zhao Y, et al. APOE hypermethylation is associated with autism spectrum disorder in a Chinese population. Exp Ther Med 2018;15:4749-54. [Crossref] [PubMed]
- Shimamoto C, Ohnishi T, Maekawa M, et al. Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum Mol Genet 2015;24:2409. [Crossref] [PubMed]
- Yamamoto Y, Kida H, Kagawa Y, et al. FABP3 in the Anterior Cingulate Cortex Modulates the Methylation Status of the Glutamic Acid Decarboxylase67 Promoter Region. J Neurosci 2018;38:10411-23. [Crossref] [PubMed]
- Herbert MR, Russo JP, Yang S, et al. Autism and environmental genomics. Neurotoxicology 2006;27:671-84. [Crossref] [PubMed]
- Mandic-Maravic V, Mitkovic-Voncina M, Pljesa-Ercegovac M, et al. Autism Spectrum Disorders and Perinatal Complications-Is Oxidative Stress the Connection? Front Psychiatry 2019;10:675. [Crossref] [PubMed]
- Benga O, Huber VJ. Brain water channel proteins in health and disease. Mol Aspects Med 2012;33:562-78. [Crossref] [PubMed]
- Duffney LJ, Zhong P, Wei J, et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep 2015;11:1400-13. [Crossref] [PubMed]
- Zhang L, Huang CC, Dai Y, et al. Symptom improvement in children with autism spectrum disorder following bumetanide administration is associated with decreased GABA/glutamate ratios. Transl Psychiatry 2020;10:9. [Crossref] [PubMed]
- Roohi J, Montagna C, Tegay DH, et al. Disruption of contactin 4 in three subjects with autism spectrum disorder. J Med Genet 2009;46:176-82. [Crossref] [PubMed]
- Yoo HJ, Kim K, Kim IH, et al. Whole exome sequencing for a patient with Rubinstein-Taybi syndrome reveals de novo variants besides an overt CREBBP mutation. Int J Mol Sci 2015;16:5697-713. [Crossref] [PubMed]
- McFadden K, Minshew NJ. Evidence for dysregulation of axonal growth and guidance in the etiology of ASD. Front Hum Neurosci 2013;7:671. [Crossref] [PubMed]
- Krueger DD, Brose N. Evidence for a common endocannabinoid-related pathomechanism in autism spectrum disorders. Neuron 2013;78:408-10. [Crossref] [PubMed]
- Zamberletti E, Gabaglio M, Parolaro D. The Endocannabinoid System and Autism Spectrum Disorders: Insights from Animal Models. Int J Mol Sci 2017;18:1916. [Crossref] [PubMed]
- Zhou J, Parada LF. PTEN signaling in autism spectrum disorders. Curr Opin Neurobiol 2012;22:873-9. [Crossref] [PubMed]
- Guang S, Pang N, Deng X, et al. Synaptopathology Involved in Autism Spectrum Disorder. Front Cell Neurosci 2018;12:470. [Crossref] [PubMed]
- Ashwood P, Differential T. Cell Levels of Tumor Necrosis Factor Receptor-II in Children with Autism. Front Psychiatry 2018;9:543. [Crossref] [PubMed]
- Parikshak NN, Swarup V, Belgard TG, et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 2016;540:423-7. [Crossref] [PubMed]


