
Characteristics and outcomes of glomerulonephritis with membranoproliferative pattern in children
Introduction
Membranoproliferative glomerulonephritis (MPGN) has been recognized as a rare pathological pattern of glomerulopathy clinically characterized by proteinuria, hematuria, hypertension and often impaired renal function at disease onset. It accounts for approximately 1–7% of all cases of biopsy-confirmed glomerulonephritis among all ages (1-4). Recent studies had revealed the incidence of this pattern was significantly declined in the 21st century, however, rates of progression to ESRD and death remained unimproved (4,5). In up to 50% of the affected children, MPGN leads to end-stage renal disease (ESRD) within ten years (6).
The typical features of MPGN on light microscopy (LM) include mesangial cellularity, endocapillary proliferation, and capillary-wall remodeling (with the formation of double contours), and lobular accentuation of glomerular tufts. Based on the electron-microscopical findings, MPGN is traditionally classified as primary (idiopathic) MPGN type I (MPGN I, with subendothelial deposits), type II (MPGN II, with dense deposits in the glomerular basement membrane), or type III (MPGN III both subepithelial and subendothelial deposits) or secondary MPGN (7). However, this kind of assortment neither indicates the etiology of MPGN, nor provides competent evidence for subsequent treatments. Lately, abnormal activation of complement via alternative pathway was found to mediate the formation of MPGN pattern (8). In 2013, an expert consensus was established and proposed a practical approach to view MPGN as immune-complex-mediated MPGN (IC-MPGN) and C3 glomerulopathy (C3G) based on immunofluorescence (IF) of renal biopsies (9). C3G was recognized by the new classification as a distinct type from MPGN pattern and further subdivided into dense deposit disease (DDD) and C3 glomerulonephritis (C3GN), depending on the position of electron-microscopical deposits. Although IC-MPGN and C3G were distinguished in histopathology, the essential borderline and interrelation between these two entities are still ambiguous.
Several studies were done to evaluate the causes, clinical presentations, effects of various treatments and prognosis of adult MPGN (10-14), yet studies of children MPGN remain to be small-scale with narrow cases (15-17). The rareness of the disease as well the terminology shift with the revolution of diagnostic classification conceal the authentic characteristics and outcomes from being concluded. Consequently, effective therapies of MPGN pattern and ameliorated prognosis have not been made further. The current therapies for MPGN including steroids and immunosuppressants suggested by KDIGO guidelines (18) have not shown consistent benefits and the evidence for therapeutic efficacy in children was extremely limited. Moreover, the latest classification of IC-MPGN and C3G remained to be evaluated on its utility in children with MPGN. Therefore, we aim to retrospectively analyze the clinical, pathological and pathogenic diagnosis of MPGN in children to provide an optimized strategy for early diagnosis of MPGN. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tp-21-286).
Methods
Study design and participants
The children with pathologic diagnosis as MPGN, aged from birth to 18 years old were enrolled at Children’s Hospital of Fudan University between January 1, 2007 and May 31, 2020. Following informed consent, we collected clinical records among the individuals with MPGN. A retrospective analysis of clinical features, pathological findings, genetic detection and renal outcome was performed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board (IRB) of Children’s Hospital of Fudan University (No. 2018_286) and informed consent was taken from all the patients’ parents.
Measurements and variable definitions
The definition of clinical phenotype and remission was shown in Table 1. Clinical data included gender, age of onset, initial presentation, treatment and outcomes were summarized in Table 2. All biopsies were prepared by standard techniques for LM, IF, and electron microscopy (EM), where available. Hematoxylin and eosin (HE) stain and periodic acid-silver methenamine (PASM) stain were applied for LM. The intensity of immunofluorescent staining for IgG, IgA, IgM, C3, C4, C1q, C3d, C9 and Fb was graded from negative to 3+ on IF. Distribution, type and extent of deposits were recorded on EM. All renal diseases were diagnosed based on the KDIGO guideline (18,19). Clinicians were asked to review the medical records and kidney biopsy findings of patients diagnosed with MPGN followed up for at least 3 months. Biopsy findings of patients diagnosed with MPGN or post infectious glomerulonephritis before the publication of the new C3G classification criteria were re-evaluated and patients meeting the new C3G criteria were included in the study. All patients were subsequently classified as IC-MPGN or C3G (including C3GN and DDD). The current criterion for C3G is based on the IF criteria of predominant glomerular C3 intensity of ≥2 levels of magnitude greater than any other immune reactant (20,21). The definition of unclassifiable MPGN (U-MGPN) involves negative stating of complement or immunoglobin (partially due to poor quality staining) in renal species. Outcomes of patients were divided into complete remission group, partial remission group and non-response group (15). All patients were stratified by renal biopsy timing. The early diagnosis group was defined as biopsy performed within six months of the disease onset. Delayed diagnosis group was defined as biopsy performed over six months after the disease onset (Table 1).
Table 1
| Term | Interpretation |
|---|---|
| Current criterion for C3G | Immunostaining reveals C3 dominance and ≥2 orders of intensity greater than any combination of IgG, IgM, IgA, and C1q |
| Complete remission | eGFR ≥90 mL/min·1.73 m2 |
| 24-hour urinary protein<0.2 g or negative/trace in dipstick | |
| Hematuria <5/Hp or negative/trace in dipstick | |
| Partial remission | eGFR ≥90 mL/min·1.73 m2 |
| Reduction of proteinuria of more than 50% compared to highest value (reduction more than 50% of 24-hour urinary protein, or decrease at least 2 order in dipstick) With or without hematuria | |
| Non-remission | Lack of complete or partial response |
| Early diagnosis | Renal biopsy was performed within 6 months after disease onset |
| Delayed diagnosis | Renal biopsy was performed over 6 months after disease onset |
| Nephrotic syndrome | Edema, proteinuria (24-hour urinary protein >50 mg/kg), hypoalbuminemia (<30 g/L) |
| Nephritic syndrome | Macroscopic hematuria, edema, hypertension, abnormal function |
| Nephritic-nephrotic syndrome | Joint occurrence of nephrotic and nephritic syndrome |
C3G, C3 glomerulopathy; eGFR was calculated by the Schwartz formula using a local k-factor of 49 in CKD 1-2, 36.5 in CKD 3-5.
Table 2
| ID | Causes | Pathological diagnosis | At disease onset | Treatment | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presentation | C3 (g/L) | C3NeF | eGFR | Steroids | MMF | CTX | ACEI | Response for steroids | Remission or not | |||||
| 1 | HBV infection | IC-MPGN | Nephritic nephrotic syndrome | 1.45 | NA | 37.2 | + | + | Resistant | Non-response, ESRD, deceased | ||||
| 2 | IC-MPGN | Nephrotic syndrome with hematuria | 0.91 | No abnormal | 111.6 | + | + | Resistant | Partial remission | |||||
| 3 | IC-MPGN | Nephrotic syndrome with hematuria | 0.28 | Abnormal | 79.8 | + | + | + | + | Resistant | Complete remission | |||
| 4 | HBV infection | IC-MPGN | Nephritic nephrotic syndrome | 0.06 | No abnormal | 109.5 | + | + | Sensitive | Complete remission | ||||
| 5 | Aymé-Gripp Syndrome MAF: p.Ser54Leu (de novo) | U-MPGN | Asymptomatic hemoglobinuria | 0.88 | NA | 138.9 | + | / | Non-response | |||||
| 6 | MMA MMACHC: p.Trp203Ter (het;p,wt; m,het); p.Gln27Arg (het;p,het; m,wt) | IC-MPGN | Nephritic syndrome | 1.29 | NA | 78.6 | + | + | + | Resistant | Non-response | |||
| 7 | IC-MPGN | Nephritic syndrome | 1.54 | NA | 131.4 | + | + | Resistant | Partial remission | |||||
| 8 | IC-MPGN | Nephrotic syndrome with hematuria | 0.15 | NA | 194.7 | + | + | Resistant | Non-response | |||||
| 9 | IC-MPGN | Nephrotic syndrome with hematuria | 0.74 | NA | 96.2 | + | + | + | Resistant | Complete remission | ||||
| 10 | HBV infection | IC-MPGN | Nephrotic syndrome with hematuria | 1.48 | NA | 104 | + | / | Complete remission | |||||
| 11 | HBV infection | U-MPGN | Asymptomatic hemoglobinuria | NA | NA | NA | + | Resistant | Non-response | |||||
| 12 | IC-MPGN | Nephritic nephrotic syndrome | 1.74 | NA | 103.9 | + | + | Resistant | Complete remission | |||||
| 13 | MMA MMACHC: p.Trp203Ter (het;p.het;m,wt) | C3G | Nephritic nephrotic syndrome | 1.21 | No abnormal | 21.4 | + | + | Resistant | Non-response, ESRD Transplantation without relapse | ||||
| 14 | IC-MPGN | Nephritic nephrotic syndrome | 0.13 | Abnormal | 102.6 | + | + | + | Resistant | Complete remission | ||||
| 15 | U-MPGN | Nephrotic syndrome with CKD3 | 0.87 | NA | 47 | + | + | Resistant | Non-response | |||||
| 16 | IC-MPGN | Nephritic nephrotic syndrome | 2.15 | NA | 79.3 | + | + | Resistant | Non-response | |||||
| 17 | RA | U-MPGN | Nephritic syndrome | 1.40 | NA | 47 | + | + | + | + | Resistant | Non-response | ||
ACEI, angiotensin converting enzyme inhibitor; C3G, C3 glomerulopathy; C3NeF, C3 nephritic factor; CTX, Cyclophosphamide; ESRD, end stage of renal disease; HBV, hepatitis B virus; IC-MPGN, immune-complex mediated membranoproliferative glomerulonephritis; MAF, MAF BZIP Transcription Factor; MMA, methylmalonic acidemia; MMACHC, Metabolism Of Cobalamin Associated C; MMF, mycophenolate mofetil; NA, no data; RA, rheumatoid arthritis, U-MPGN, unclassifiable membranoproliferative glomerulonephritis; eGFR was calculated by the Schwartz formula using a local k-factor of 49 in CKD 1-2, 36.5 in CKD 3-5.
Statistical analysis
Data were analyzed using Excel. Continuous variables were summarized with median, IQR and categorical data were summarized with proportions. Mann-Whitney U test (for continuous variables that do not conform to the normal distribution and homoscedasticity) and the Fisher exact probability test (for categorical variables) were used to analyze the differences among participants with different clinical features stratified into early or delayed diagnosis groups. Spearman correlation analysis was performed to assess the correlation between serum C3 and proteinuria during the follow-up. Statistical analysis was performed with SPSS version 25.0 statistical package software (IBM Co., Armonk, NY, USA). Figures were performed using GraphPad Prism 7.0.
Results
A total of 17 children (ten boys and seven girls) with MPGN were recruited from the 1,901 (1.4%) cases with complete clinical and pathological records of the children’s hospital of Fudan University from 2007 to 2020. The median age at onset was 9.9 years (IQR, 5.6–11.9 years) with a median eGFR of 102.6 mL/min·1.73 m2 (IQR, 47.0–121.5 mL/min·1.73 m2) at the time of admission.
Clinical presentation at diagnosis and underlying causes
Clinical and biological data for the patients at diagnosis are summarized in Table 2 and Table S1. Prior infections were reported in nearly half of the patients (n=8), including upper respiratory tract infections (n=4) and skin infections (n=4) (Table S1). In our study, most of the patients (12/17) had nephrotic range of proteinuria, and nephritic-nephrotic syndrome was the most common clinical presentation (Table 2). Fifteen (88.2%, 15/17) patients had microscopic or macroscopic hematuria at onset. Hypocomplementemia was present in six (35.3%, 6/17) children including isolated low serum C3 values (n=4), isolated low serum C4 value (n=1), and low serum C3 and C4 together (n=1). Hypertension and decline in kidney function were observed in four and seven patients at onset, respectively. Four patients had a history of familial glomerulonephritis (Table S2).
Underlying causes were found in a high proportion (8/17, 47.1%) of our MPGN children (Table 2). Diagnosis of IC-MPGN makes up a larger proportion in our patients (12/17), and no DDD case was found in our patients, which is quite different from other studies mainly comprised by C3G (15), especially DDD (14). It may due to the high incidence of hepatitis B virus infection in China. Four (23.5%) patients were found to be secondary to hepatitis B virus infection and most of them presented with IC-MPGN. In addition to hepatitis B virus infection, MMA is another cause of MPGN. Two patients were diagnosed with MMA based on genetic analysis when developing into chronic kidney disease (CKD), and the prognosis was not as good as the other children. Standard therapy of vitamin B12 injections in responsive MMA patients should be started at an early stage. It suggests that more attention to paid to screening for MMA in childhood MPGN.
Pathological findings and pathogenic diagnosis
IF staining of renal biopsies was summarized in Figure 1. Three patients with HBV-associated MPGN showed IC-MPGN, and one patient with HBV-associated MPGN showed U-MPGN. One patient with MMA presented as IC-MPGN, and the other one with MMA showed C3G. One patient with rheumatoid arthritis (RA) was recorded as U-MPGN. Base on the current criterion, only one patient (patient #13) secondary to MMA was diagnosed as C3G with C3 dominance of two orders of magnitude stronger than any other immune reactant (20). However, we failed to establish the diagnosis of C3G for the other four patients (patient #3, #7, #8 and #14) with C3 dominance of one order intensity greater than any other immune reactant by IF staining of renal biopsies. Three of the four patients had obvious activation of the alternative pathway with an abnormal level of C3 nephritic factor (C3NeF) or C3 (Table S2). None was diagnosed as DDD based on electron density deposition under the electron microscope. Therefore, the nine patients with primary MPGN were further identified as IC-MPGN (n=8) and unclassifiable MPGN (U-MGPN, n=1) (Table 2). Repeated renal biopsy was performed in patient #3 because of relapse.
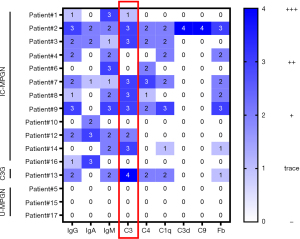
At initial presentation, low serum C3 values (normal 0.67–1.76 g/L) were presented in patients with C3G (n=1) and IC-MPGN (n=4) (Table 2). Screening for the presence of C3NeF was performed in five patients. Abnormal level of C3NeF were reported in two patients at onset (Table 2). Exome sequencing was performed in five patients considering the extrarenal phenotypes and C3 dominant deposit by immunofluorescence staining of renal biopsies with suspicion of congenital complement disorder. No pathogenic variations were identified in the complement regulatory genes. Compound heterogeneous variants of MMACHC were identified in one of the patients who were clinical suspicion of MMA with homocysteinemia.
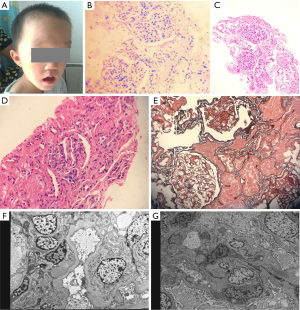
One patient (patient #5) was diagnosed with deafness, bilateral cataracts and intellectual disability within one year old who present non-nephrotic range proteinuria at age of eight years old. Serum creatinine was 26 µmol/L, albumin 42.1 g/L, C3 was 0.88 (0.67–1.76 g/L). Serologically he had negative ANA, pANCA/cANCA and dsDNA. Ultrasonography showed the normal size of kidneys with maintained cortical medullary differentiation. Kidney biopsy revealed MPGN without any deposits of immune reactant by immunofluorescence or electron microscope (Figure 2). In view of the multisystem phenotypes, we performed trio-exome sequencing identifying a de novo heterogeneous variant (p.Ser54Leu) of MAF gene which is the known molecular cause for Aymé-Gripp syndrome.
Treatments and outcomes
The median follow-up was 2.4 years (IQR, 1.0–4.5 years). Nearly half (47.3%, 8/17) of them were followed more than 3 years. We can see a high proportion of patients (14/15, 93.3%) did not respond to the initial therapy of steroids. Therefore, additional immunosuppressive regimens were used in most of these patients to induce clinical remission (Table 2). The combination of steroids with MMF or CTX induced complete remission in four patients with poor response to initial steroids therapy. Complications of treatments were observed in eight patients during the follow-up, including hypertension (n=3), dental ulcer (n=2), cataract (n=1), blurred vision (n=1), high intraocular pressure (n=1) and urinary tract infection (n=3). Some patients had more than one complication. All the details could be found in Table S2.
At the last follow-up, nearly half of the patients responded well to the interventions and eight of them developed complete or partial remission (Figure 3). Although receiving aggressive therapies, two patients progressed to ESRD 10.5 months and 4.7 years after disease onset respectively, and one of them deceased within a year post ESRD. Since nearly half of our patients with MPGN do not respond well to routine treatments, it is important to explore the factors associated with renal prognosis. We analyzed clinical factors including the etiology, disease onset age, treatments, family history, the timing of pathological diagnosis and extrarenal manifestations (Table 3). There were more patients who achieve full or partial remission in the early diagnosis patient group compared with the patients from the delayed diagnosis patient group (P<0.05, Table 3). The relationship between the timing of renal biopsy and outcomes were further marked in Figure 3. All patients received early diagnosis had achieved a complete or partial remission at the last follow-up, except for those patients with secondary MGPN caused by MMA and Aymé-Gripp Syndrome.
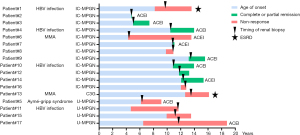
Table 3
| Variables | Total | Complete or partial remission | Non-response | P value |
|---|---|---|---|---|
| Number | 17 | 8 | 9 | / |
| Treatments | ||||
| Prednisolone | 15 | 7 | 8 | 1.000 |
| Steroid resistant | 14 | 6 | 8 | 0.576 |
| Immunosuppressants | 11 | 4 | 7 | 0.131 |
| MMF | 7 | 3 | 4 | 1.000 |
| CTX | 5 | 1 | 4 | 0.294 |
| ACEI | 10 | 7 | 3 | 0.05 |
| Complications | 8 | 4 | 4 | 1.000 |
| Factors | ||||
| Gender (female) | 7 | 4 | 3 | 0.637 |
| Age onset (years) | 9.9 (5.6, 11.9) | 10.8 (6.4, 12.0) | 8.2 (5.4, 11.0) | 0.309 |
| Follow-up (years) | 2.4 (1.0, 4.5) | 1.6 (0.3, 2.4) | 3.6 (2.1, 8.2) | 0.023 |
| Initial eGFR (mL/min/1.73 m2) | 102.6 (47.0, 121.5) | 106.8 (103.0, 126.4) | 47.0 (35.5, 109.1) | 0.092 |
| Initial proteinuria (24 hours urine protein quantification) | 2.6 (1.5, 5.4) | 2.7 (1.8, 3.8) | 2.5 (1.0, 7.2) | 0.378 |
| Primary MPGN | 9 | 6 | 3 | 0.153 |
| Other renal/extrarenal manifestations | 3 | 0 | 3 | 0.206 |
| Positive family history of kidney disease | 4 | 1 | 3 | 0.576 |
| Early diagnosis | 10 | 8 | 2 | 0.002 |
| Delayed diagnosis | 7 | 0 | 7 |
eGFR was calculated by the Schwartz formula using a local k-factor of 49 in CKD 1-2, 36.5 in CKD 3-5. Data are given as median (interquartile range). ACEI, angiotensin converting enzyme inhibitor; CTX, Cyclophosphamide; MMF, mycophenolate mofetil; MPGN, membranoproliferative glomerulonephritis.
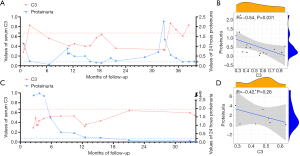
Although consistent reduced serum C3 value was considered as a sign for activation of the alternative complement pathway, the relationship between serum C3 level and proteinuria in MPGN patients was still undetermined. Therefore, we analyzed the correlation between serum C3 levels and 24-hour urinary protein during the follow-up of two patients (patient #3 and #14) with positive C3NeF and detailed information during follow-up (Figure 4). Patient #3 presented with nephrotic-range proteinuria, hematuria and abnormal kidney function at disease onset (Table S1). Complement tests showed a low level of serum C3 levels and elevated C3NeF. Steroids and RAAS blockers were started. A partial remission with decreasing proteinuria was showed, whereas the C3 level remained low after eight weeks of steroids therapy. Because of recurrent proteinuria when steroids tapering accompanied by a skin infection, mycophenolate mofetil (MMF) was started in combination with steroids. After a twenty-month remission, proteinuria subsequently relapsed again when reducing the dosage of MMF. Then, MMF was stopped and shifted to cyclophosphamide (CTX). Eight months after starting CTX, a significant decline of proteinuria and increased serum C3 were observed with stabilization of eGFR. Patient #14 presented with nephritic-nephrotic syndrome and normal renal function at the onset. After a poor response to steroids initially, she started MMF. Complete remission of proteinuria and maintained normal renal function were achieved with successfully tapering steroids and MMF, whereas serum C3 remained persistently low value. We can see a negative relationship between serum C3 value and urine protein (Figure 4), whereas serum C3 value and C3NeF level was not found correlatedly (Figure S1).
Discussion
This study presented the clinical features of 17 children with MPGN of kidney injury in a single center in China providing evidences for diagnosis and outcomes for childhood MPGN. It is essential to identify the diagnosis in MPGN concerning the pathological classification, secondary etiology and genetic causes. A previous study had reported lupus nephritis was the most common cause among adult patients with secondary MPGN (12), and our results presented HBV infection as the most common cause for secondary MPGN in pediatric patients. A heterogenous clinical presentation of our patients displayed as nephritic-nephrotic syndrome, nephrotic syndrome with hematuria or abnormal renal function, nephritic syndrome and asymptomatic hematuria or proteinuria, in line with the previous reports (22,23).
Genetic detection helps to explore the underlying causes of MPGN. The invariable presence of C3 in the glomerulus has implicated complement alternative pathway activation as a key causal mechanism and testing for complement gene mutations is currently recommended in C3G. It has been documented by a study of 146 European cases and 6,442 European controls that there is no association of rare complement gene variants with primary MPGN (24). Although we did not observe rare pathological variants in the candidate genes (encoding components of the complement alternative pathway), further analysis on common alleles of the other genes may show possible causal mechanisms for MPGN. Besides the genetic findings in MMACHC gene to support the diagnosis of MMA, we also identify the de novo variant of MAF gene (p.Ser54Leu) for Aymé-Gripp syndrome. Dominant pathogenic variants of MAF (MIM# 601088) are associated with Aymé-Gripp syndrome, a condition presenting with mild to severe intellectual disability syndrome, autism spectrum disorder, cataracts, short stature, seizures, and skeletal involvement. To date, there were only two adult cases of Aymé-Gripp syndrome present late-onset renal disease (25,26). It has been reported a 43-year-old patient with the same variant of MAF (p.Ser54Leu) presented with proteinuria and typical clinical features of Aymé-Gripp syndrome, subsequently diagnosed with mesangiocapillary glomerulopathy (26). Our findings suggest that renal manifestations of MPGN could also present in children with Aymé-Gripp syndrome and add clinical value in monitoring patients with MAF pathogenic variants for changes in renal function.
A new proposed diagnostic standard of C3G was C3 dominant at least two orders of magnitude more intense than any other immune reactant, which requires validation by alternative pathway evaluation (20,21). We applied this standard in our pediatric patients and found two patients with obvious alternative pathway evaluation would be failed to identify the diagnosis of C3G in this condition. Similar situations were also observed in other pediatric MPGN studies (15,16). Accordingly, there do exist several pediatric MPGN patients with dysregulation of alternative pathway who do not conform to the “C3 dominant at least two orders of magnitude more intense than any other immune reactant” criterion. It seems that current diagnostic criteria of IF labeling for C3G may be strict on pediatric patients with MPGN. Previous studies had shown the prognosis of patients with C3G is worse than patients with IC-MPGN, particularly in DDD patients (23,27). Therefore, we must take a close look at the clinical course for the patients with C3 dominant deposit in the glomerulus. Outcome-related factors were also analyzed in this study. We discovered most patients who received early diagnosis reached complete or partial remission at the last follow-up, whereas patients who received delayed diagnosis all turned out non-response for usual treatments. It suggests that early pathological diagnosis in children may help to optimize the treatment and prevent the decline of renal function. Although there are controversial results, it has recently been shown that the treatment of MPGN with corticosteroids plus MMF in adults caused better kidney survival as compared with patients treated with other immunosuppressants and untreated patients (28).
Limitations of our study include the following: First, as a retrospective observational study, we were unable to perform genetic and serological tests in all patients, and therefore, evaluation of the possible effects of specific genetic or serological markers was not possible. Second, our study was small case series from a single-center study collecting the clinical data from 2007 to 2020. MPGN is rare, with an incidence estimated at 1–2 per million population (29). A large pediatric cohort of MPGN should be carried out in multiply medical centers from different regions. Third, long-term follow-up needs to be performed in this rare disease.
Conclusions
We present detailed valuable information on the pediatric case series of MPGN. Integrated analysis of genotype and phenotype including pathological and clinical findings is pivotal for early identification of the primary or secondary causes of MPGN in children.
Acknowledgments
Funding: JR is supported by a grant from National Natural Science Foundation of China (NSFC-8182207), a grant from Program of Shanghai Academic/Technology Research Leader (19XD1420600).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-286
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tp-21-286
Peer Review File: Available at https://dx.doi.org/10.21037/tp-21-286
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-286). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board (IRB) of Children’s Hospital of Fudan University (No. 2018_286) and informed consent was taken from all the patients’ parents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol 2006;1:483-7. [Crossref] [PubMed]
- Zhou FD, Zhao MH, Zou WZ, et al. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 2009;24:870-6. [Crossref] [PubMed]
- Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 2001;16:1364-7. [Crossref] [PubMed]
- O'Shaughnessy MM, Hogan SL, Poulton CJ, et al. Temporal and Demographic Trends in Glomerular Disease Epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol 2017;12:614-23. [Crossref] [PubMed]
- Heaf JG, Sørensen SS, Hansen A. Increased incidence and improved prognosis of glomerulonephritis: a national 30-year study. Clin Kidney J 2020;14:1594-602. [Crossref] [PubMed]
- Smith RJ, Alexander J, Barlow PN, et al. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 2007;18:2447-56. [Crossref] [PubMed]
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 2012;366:1119-31. [Crossref] [PubMed]
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol 2011;31:341-8. [Crossref] [PubMed]
- Masani N, Jhaveri KD, Fishbane S. Update on membranoproliferative GN. Clin J Am Soc Nephrol 2014;9:600-8. [Crossref] [PubMed]
- Nakano M, Karasawa K, Moriyama T, et al. Characteristics of membranoproliferative glomerulonephritis based on a new classification at a single center. Clin Exp Nephrol 2019;23:852-8. [Crossref] [PubMed]
- Wilson GJ, Cho Y, Teixiera-Pinto A, et al. Long-term outcomes of patients with end-stage kidney disease due to membranoproliferative glomerulonephritis: an ANZDATA registry study. BMC Nephrol 2019;20:417. [Crossref] [PubMed]
- Nakagawa N, Hasebe N, Hattori M, et al. Clinical features and pathogenesis of membranoproliferative glomerulonephritis: a nationwide analysis of the Japan renal biopsy registry from 2007 to 2015. Clin Exp Nephrol 2018;22:797-807. [Crossref] [PubMed]
- Iatropoulos P, Daina E, Curreri M, et al. Cluster Analysis Identifies Distinct Pathogenetic Patterns in C3 Glomerulopathies/Immune Complex-Mediated Membranoproliferative GN. J Am Soc Nephrol 2018;29:283-94. [Crossref] [PubMed]
- Lu Y, Shen P, Li X, et al. Re-evaluation of the classification system for membranoproliferative glomerulonephritis. Contrib Nephrol 2013;181:175-84. [Crossref] [PubMed]
- Holle J, Berenberg-Goßler L, Wu K, et al. Outcome of membranoproliferative glomerulonephritis and C3-glomerulopathy in children and adolescents. Pediatr Nephrol 2018;33:2289-98. [Crossref] [PubMed]
- Spartà G, Gaspert A, Neuhaus TJ, et al. Membranoproliferative glomerulonephritis and C3 glomerulopathy in children: change in treatment modality? A report of a case series. Clin Kidney J 2018;11:479-90. [Crossref] [PubMed]
- Okuda Y, Ishikura K, Hamada R, et al. Membranoproliferative glomerulonephritis and C3 glomerulonephritis: frequency, clinical features, and outcome in children. Nephrology (Carlton) 2015;20:286-92. [Crossref] [PubMed]
- Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines--application to the individual patient. Kidney Int 2012;82:840-56. [Crossref] [PubMed]
- Floege J, Barbour SJ, Cattran DC, et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019;95:268-80. [Crossref] [PubMed]
- Pickering MC, D'Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int 2013;84:1079-89. [Crossref] [PubMed]
- Hou J, Markowitz GS, Bomback AS, et al. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 2014;85:450-6. [Crossref] [PubMed]
- Çaltik Yilmaz A, Aydog Ö, Akyüz SG, et al. The relation between treatment and prognosis of childhood membranoproliferative glomerulonephritis. Ren Fail 2014;36:1221-5. [Crossref] [PubMed]
- Khandelwal P, Bhardwaj S, Singh G, et al. Therapy and outcomes of C3 glomerulopathy and immune-complex membranoproliferative glomerulonephritis. Pediatr Nephrol 2021;36:591-600. [Crossref] [PubMed]
- Levine AP, Chan MMY, Sadeghi-Alavijeh O, et al. Large-Scale Whole-Genome Sequencing Reveals the Genetic Architecture of Primary Membranoproliferative GN and C3 Glomerulopathy. J Am Soc Nephrol 2020;31:365-73. [Crossref] [PubMed]
- Alkhunaizi E, Koenekoop RK, Saint-Martin C, et al. Maternally inherited MAF variant associated with variable expression of Aymé-Gripp syndrome. Am J Med Genet A 2019;179:2233-6. [Crossref] [PubMed]
- Niceta M, Stellacci E, Gripp KW, et al. Mutations Impairing GSK3-Mediated MAF Phosphorylation Cause Cataract, Deafness, Intellectual Disability, Seizures, and a Down Syndrome-like Facies. Am J Hum Genet 2015;96:816-25. [Crossref] [PubMed]
- Kawasaki Y, Kanno S, Ono A, et al. Differences in clinical findings, pathology, and outcomes between C3 glomerulonephritis and membranoproliferative glomerulonephritis. Pediatr Nephrol 2016;31:1091-9. [Crossref] [PubMed]
- Kirpalani A, Jawa N, Smoyer WE, et al. Long-Term Outcomes of C3 Glomerulopathy and Immune-Complex Membranoproliferative Glomerulonephritis in Children. Kidney Int Rep 2020;5:2313-24. [Crossref] [PubMed]
- McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414-30. [Crossref] [PubMed]


