
The optimal dose of oral midazolam with or without intranasal S-ketamine for premedication in children: a randomised, double blinded, sequential dose-finding trial
Introduction
Premedication is used to reduce the pre-operative stress on the children and their parents, also ease the separation from each other. A number of methods and medicines are reported to be used in premedication for children, among which oral administration of midazolam syrup is one of the most favorable methods (1-5).
Rapid onset, relatively short duration of action, anterograde amnesia, lack of significant side effects makes midazolam a most favorable choice for premedication by anesthesiologists (1-3). However, several side effects of midazolam including paradoxical reactions, interactions with opioids, excessive sedation, disorientation, and impaired psychomotor performance have been reported (1,6-8). S-ketamine, a S(+)-enantiomer of ketamine, has a more highly efficient analgesic and sedative effect with less severe side effects for children as compared to ketamine (9).
We hypothesis intranasal small dose of S-ketamine could significantly reduce the need of oral midazolam syrup without obvious side effects. The aim of this study was to estimate the 90% effective dose (ED90) of oral midazolam syrup with and without intranasal low dose of S-ketamine in the premedication for preschool children, and to further compare the effect and side effect between two methods.
We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/tp-21-247).
Methods
Materials and methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Research Ethics Committee of Shanghai Child Medical Centre (K2020059-2) and registering at http://www.chictr.org.cn (ChiCTR2100044635), the parents or legal guardians from each participant provided written informed consent for participation in this study.
A total of 85 children aged 2–6 years, with American Society of Anesthesiology (ASA) scored I–II, undergoing elective surgeries with general anesthesia were recruited during the study period from April 2021 to May 2021 in Shanghai Child Medical Center.
Exclusion criteria
- Children with ASA score of III–IV;
- Allergy to midazolam, S-ketamine or ketamine;
- Children with a history of below:
- Upper airway disease;
- Central nervous system dysfunction;
- Cardiovascular dysfunction;
- Gastrointestinal disorders;
- Intranasal pathology or running nose.
- Surgery time ≥90 min;
- Obesity with a body weight over 30 kg;
- Emergent surgery;
- Children or parents refusal.
Preoperative follow-up
The preoperative follow-up included a detailed history, physical examination of child, and review of investigation, was performed the day before surgery. Also stopping intake clear fluids for 4 hours or solid and milk for 6 hours was suggested to the parents.
Trial design
This was a prospective, double-blind, randomised, biased coin up-and-down (BCUD), sequential dose-finding trial. After providing signed informed consent from the parents, children were randomly allocated into two groups according to the randomized numbers generated by a research assistant using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA): the oral midazolam syrup group (M group) and the oral midazolam syrup combined with intranasal S-ketamine group (MS group), with 40 children each group. All the anesthesiologists, nurses, children and their parents were blinded to the drugs and doses used.
Technique
M group (n=40): the children were given research dose of oral midazolam syrup according to the body weight and then intranasal normal saline 0.3 mL 30 min before entering operation room (OR) by a fixed nurse anesthetist.
MS group (n=40): the children were given research dose of oral midazolam syrup according to the body weight and then intranasal S-ketamine 0.25 mg/kg with normal saline diluted to a volume of 0.3 mL 30 min before entering OR by the nurse anesthetist.
A midazolam dose of 0.25 mg/kg was used as the first dose of each group (10). The dose of midazolam for subsequent children was determined by the response of each previous child. Satisfactory sedative effect at separation was defined as a parental separation anxiety score (PSAS) =1 during the transferring from premedication center to OR; if satisfactory PSAS achieved, the dose of midazolam was considered a success, and the next patient was randomly assigned a dose with a 1/9th chance of receiving a lower dose (decreased by 0.05 mg/kg) or an 8/9th chance of receiving the same dose as the previous child. Otherwise, the midazolam dose was considered failed and the dose for the following child was increased by 0.05 mg/kg. The floor and the ceiling dose of oral midazolam dose were 0.2 and 0.6 mg/kg separately. All the oral midazolam dose for 40 successive child of both groups was implemented using the BCUD scheme prepared by a study statistician in Microsoft Excel 2016, and was used by the research assistant, who was the only person with access to this software, preparing the drugs for medication, maintaining the double-blind nature of the study.
Routing monitoring, including electrocardiograph, non-invasive blood pressure, and pulse oximetry, was performed continuously before premedication. The systemic arterial pressure, heart rate (HR), level of sedation, and side effects of medication were recorded every 5 min. The duration from the medication to the time of the child complaining dizziness or drowsy, or the anesthesiologist scoring ≥3 in level of sedation was regarded as the onset time. Thirty minutes after the premedication, the child was taken into the OR on transferring bed, PSAS score was assessed. After transferred to surgical bed, routing monitoring including electrocardiograph, non-invasive blood pressure, and pulse oximetry was performed continuously. One hundred percent oxygen was given via facemask and mask acceptance by the child was noted. Then an intravenous (IV) line was established by 24G cannulation and reaction to IV cannulation was assessed. If the child doesn’t cooperate with IV line establishment, 8% sevoflurane was inhaled through mask. Propofol 2.5 mg/kg, fentanyl 2 µg/kg and rocuronium 0.6 mg/kg were used to induce the anesthesia after IV cannulation. Two minutes after the induction of anesthesia and preoxygenation, the endotracheal intubation was conducted. The pressure-controlled ventilation mode with a peak pressure of 12–14 cmH2O and a rate of 15 times per min was used. The rate was adjusted to maintain the value of end tidal CO2 within 35–40 mmHg. Propofol 5 mg/kg/h and sevoflurane 0.8–1% were used to maintain the anesthesia. The infusion rate and inhalation concentration were adjusted according to the blood pressure of child during surgery. All children received ketorolac 0.5 mg/kg for post-analgesia. All maintaining drugs were stopped and disconnected at the end of surgery, and sugammadex 2 mg/kg was used to reverse neuromuscular blockade. The child was extubated after suctioning of oral cavity, achieving adequate respiratory rate and tidal volume. Then the child was transferred to post anesthesia care unit (PACU) for further observations. In PACU, the intensity of pain was scored by the Face, Legs, Activity, Cry and Consolability (FLACC) scale (ranges from 0 to 10) every 15 min. If FLACC scored ≥3, rescue analgesic drugs was administrated. The other adverse effects were also observed and recorded. When the Aldrete recovery score >8, the child could be discharged from PACU.
Parameters observed
- Level of sedation was assessed by five-point scale as follows:
- Agitated (clinging to parents, crying);
- Alert (awake may whimper not crying);
- Calm (sitting/lying comfortably with eyes open);
- Drowsy (lying comfortably with eyes closed, responds to minor stimulus);
- Asleep (eyes closed, no response to minor stimulus).
- PSAS of the child at 30 min was noted as follows:
- Easy separation;
- Whimpers, but is easily reassured, not clinging;
- Cries and cannot be easily reassured, but not clinging to parents;
- Crying and clinging to parents.
- Mask acceptance scale (MAS) was assessed by four-point Likert scale as follow:
- Excellent (unafraid, cooperative, accepts mask readily);
- Good (slight fear of mask, easily reassured);
- Fair (moderate fear of mask, not calmed with reassurance);
- Poor (terrified, crying, or combative).
- Reaction to intravenous cannulation scale (ICS) was as follows:
- Resistance without success;
- Resistance with success;
- Minor resistance;
- No reaction.
The primary outcome was the oral midazolam dose with and without intranasal small dose of S-ketamine to achieve satisfactory PSAS scores of the child at 30 min. Secondary outcomes included preoperative and post-operative observations. Preoperative observation included the systolic blood pressure (SBP), HR, level of sedation, onset time, side effects of premedication, PSAS scores at 30 min, MAS scores, ICS scores, SBP and HR before and after induction of anesthesia. Post-operative observations included extubation time, conscious recovery time, time of PACU stay, extra analgesic drug use, adverse events such as hypoxemia, nausea and vomiting. Demographics such as age, weight, height, baseline SBP, baseline HR and surgical time were also recorded.
Sample size calculation
Pace et al. suggested that the stopping rule of enrolling 20–40 patients in BCUD-based sequential dose-finding study will provide stable estimates of the target dose for most realistic cases (11). So the sample size of each group was 40 children in this study.
Statistical analysis
The ED90 was defined as the oral dose of midazolam according to the weight associated with success of the primary outcome in 90% of the study population, and it was estimated by isotonic regression method (6,11). The 95% confidence interval (CI) of isotonic regression estimator of ED90 was obtained by a bias-corrected percentile method (11) using 2,000 bootstrap replications. The isotonic regression and bootstrapping were performed by the study statistician using R version 3.4.4. (R Foundation for Statistical Computing, Vienna, Austria).
Demographic characteristics and secondary outcome estimates were reported as means ± standard deviations, median (interquartile range), numbers, and numbers (proportions). Parametric data were analyzed with the t-test; nonparametric data were analyzed with the Mann-Whitney test. Proportions were compared, using the Chi-square and Fisher exact tests, as suitable. The preoperative hemodynamic parameters were assessed by multiple t-tests and statistical significance determined using the Holm-Sidak method, with alpha =0.05. Statistical comparisons were made using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8 for windows (GraphPad Software, San Diego, California, USA). Statistical significance was defined as P values <0.05.
Results
The patient recruitment and follow-up were from April 2021 to May 2021, which is presented in Figure 1. Date from 40 children of each group were included into the analysis. The characteristics were similar between the two groups (Table 1).
Table 1
| Characteristics | M group (n=40) | MS group (n=40) | P value |
|---|---|---|---|
| Age, years | 3.8 (2.9–4.5)a | 4.3 (3.4–4.8)a | 0.084 |
| Weight, kg | 16.5 (14.8–19.0)a | 18.2 (16.3–19.5)a | 0.325 |
| Height, cm | 102.5 (95.5–109.5)a | 107 (99.3–111.8)a | 0.481 |
| Gender (male/female) | 29/11 | 27/13 | 0.808 |
| Baseline HR, bpm | 105±18 | 107±17 | 0.661 |
| Baseline SBP, mmHg | 103±12 | 106±11 | 0.182 |
| Surgical time, min | 35±23 | 32±23 | 0.524 |
Values are mean ± SD or number if not otherwise specified. a, median (IQR). M group: the oral midazolam syrup group; MS group: the oral midazolam syrup combined with intranasal S-ketamine group. HR, heart rate; SBP, systolic blood pressure; SD, standard deviation; IQR, Interquartile range.
The sequence of effective and ineffective dose of oral midazolam without and with intranasal S-ketamine in premedication in M and MS groups (Figure 2 and Figure 3 respectively). The observed and pooled-adjacent-violators algorithm-adjusted response rates associated with each used oral midazolam dose interval in the M and MS groups (Table 2 and Table 3, respectively). The ED90 dose of oral midazolam was 0.461 mg/kg (95% CI: 0.425–0.488), and the ED90 dose of oral midazolam was reduced to 0.253 mg/kg (95% CI: 0.242–0.278) when combined with intranasal S-ketamine. The ED90 dose of midazolam was reduced by approximately 45.2% (95% CI: 42.3–45.7%), when combined with 0.25 mg/kg dose of S-ketamine.
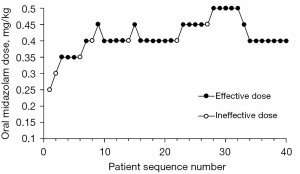
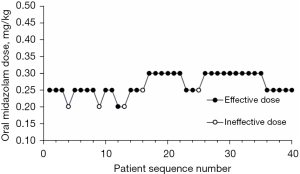
Table 2
| Assigned dose (mg/kg) | Number of successes | Number of patients | Observed response rate (%) | PAVA-adjusted response rate (%) |
|---|---|---|---|---|
| 0.25 | 0 | 1 | 0.000 | 0.000 |
| 0.30 | 0 | 1 | 0.000 | 0.000 |
| 0.35 | 3 | 4 | 0.750 | 0.750 |
| 0.40 | 18 | 21 | 0.857 | 0.857 |
| 0.45 | 7 | 8 | 0.875 | 0.875 |
| 0.50 | 5 | 5 | 1.000 | 1.000 |
PAVA-adjusted response rates were estimated using the weighted isotonic regression method. M group: the oral midazolam syrup group. PAVA, pooled-adjacent-violators algorithm.
Table 3
| Assigned dose (mg/kg) | Number of successes | Number of patients | Observed response rate (%) | PAVA-adjusted response rate (%) |
|---|---|---|---|---|
| 0.20 | 1 | 4 | 0.250 | 0.250 |
| 0.25 | 18 | 20 | 0.900 | 0.900 |
| 0.30 | 16 | 16 | 1.000 | 1.000 |
PAVA-adjusted response rates were estimated using the weighted isotonic regression method. MS group: the oral midazolam syrup combined with intranasal S-ketamine group. PAVA, pooled-adjacent-violators algorithm.
In the children’s outcomes, premedication with oral midazolam has longer onset time than that with oral midazolam and intranasal S-ketamine (19.7±7.4 vs. 8.9±3.8 min, P<0.001). The incidence of children behavioral changes was higher in M group than that in MS group (32.5% vs. 7.5%, P=0.010). Other preoperative side effects were not different between two groups (Table 4). There weren’t any differences in PSAS, MAS and ICS scores between M group and MS group. The post-operative conscious recovery (31.6±13.5 vs. 21.6±14.1 min, P=0.002) and time of PACU stay (41.5±13.6 vs. 33.5±13.5 min, P=0.010) were longer in M group than MS group; and the time of extubation, the post-operative need for rescue analgesic drug in PACU and side effects were no different between two groups (Table 4).
Table 4
| Outcomes | M group (n=40) | MS group (n=40) | P value |
|---|---|---|---|
| Preoperative outcomes | |||
| Onset (min) | 19.7±7.4 | 8.9±3.8 | <0.001 |
| Hypoxemia, n (%) | 0 (0) | 0 (0) | 1.000 |
| Hypotension, n (%) | 4 (10) | 0 (0) | 0.116 |
| Bradycardia, n (%) | 0 (0) | 0 (0) | 1.000 |
| Hypertension, n (%) | 1 (2.5) | 0 (0) | 1.000 |
| Tachycardia, n (%) | 0 (0) | 0 (0) | 1.000 |
| Nausea, n (%) | 0 (0) | 0 (0) | 1.000 |
| Vomiting, n (%) | 0 (0) | 0 (0) | 1.000 |
| Behavioral changes | 13 (32.5) | 3 (7.5) | 0.010 |
| PSAS scores [1, 2, 3, 4] | 33/1/4/2 | 35/2/2/1 | 0.741 |
| MAS scores [1, 2, 3, 4] | 29/5/0/6 | 29/8/1/2 | 0.287 |
| ICS scores [1, 2, 3, 4] | 3/6/8/23 | 1/3/14/22 | 0.332 |
| Post-operative outcomes | |||
| Extubation time, min | 7.7±3.8 | 6.4±3.4 | 0.114 |
| Conscious recovery, min | 31.6±13.5 | 21.6±14.1 | 0.002 |
| PACU stay, min | 41.5±13.6 | 33.5±13.5 | 0.010 |
| Rescue analgesic drug use, n (%) | 6 (15.0) | 3 (7.5) | 0.481 |
| Nausea, n (%) | 0 (0) | 0 (0) | 1.000 |
| Vomiting, n (%) | 0 (0) | 0 (0) | 1.000 |
| Hypoxemia, n (%) | 2 (5.0) | 0 (0) | 0.494 |
Values are numbers (percent), mean ± SD or number. M group: the oral midazolam syrup group; MS group: the oral midazolam syrup combined with intranasal S-ketamine group. PSAS, parental separation anxiety score; MAS, Mask acceptance scale; ICS, intravenous cannulation scale; PACU, post anesthesia care unit; SD, standard deviation.
There was no different in each time point of SBP between M group and MS group on serial changes of SBP over time in percent compared to baseline value before premedication (Figure 4A). The HR in 10, 15, 20, 25 min were lower in MS group than those in M group on serial changes of HR over time in percent compared to baseline value before premedication (P=0.001, 0.002, 0.003 and 0.004, respectively; Figure 4B).

Discussion
Surgery and anesthesia can induce negative emotions of children like fear, anxiety or even aggressive behaviors. Premedication is used to reduce or eliminate these emotions from the children, and also ease the separations from their parents. A number of methods and medicines are reported to be used in premedication for children, among which oral administration of midazolam syrup is one of the most favorable methods (1-5). However, there isn’t any study on the optimum dose of oral midazolam or combined it with S-ketamine in premedication for preschool children till now.
In this BCUD sequential dose-finding study, oral midazolam syrup and oral midazolam syrup combined with intranasal S-ketamine were used as premedication for preschool children, and their ED90 were estimated by Isotonic regression. The ED90 dose of oral midazolam was 0.461 mg/kg (95% CI: 0.425–0.488), and the ED90 dose of oral midazolam was reduced to 0.253 mg/kg (95% CI: 0.242–0.278) when combined with intranasal S-ketamine. The ED90 dose of midazolam was reduced by approximately 45.2% (95% CI: 42.3–45.7%), when combined with 0.25 mg/kg dose of S-ketamine.
Paradoxical reactions of midazolam including delayed recovery, anxiety, behavioral changes, agitation (6). In the study, behavioral changes were the common side effects compared with the other side effects during 30 min after the premedication. The children developed the symptom of hallucinations, or some strange behavioral changes happened. But it seemed that this side effect wasn’t associated with the dosage of midazolam, since it also happened in the cases with low dose midazolam administration. But in MS group, the incidence of behavioral changes was much lower. This may attribute to the combination of S-ketamine, and Golparvar et al. demonstrated small dose of ketamine 0.5 mg/kg could rapidly calm down the paradoxical reaction following intravenous midazolam premedication in pediatric patients (12). Furthermore, several studies proved that the combination midazolam with ketamine provided a better sedative effect for premedication in children than they used individually, which reduced the doses of both drugs and the incidences of side effect (10,13-16). Weber et al. also demonstrated the premedication with S-ketamine and midazolam provided good conditions for induction of anesthesia in preschool children (17), which had the same conclusions with the current study. However, in the procedure of sedation for magnetic resonance imaging (MRI) in children, the combination of sedative drugs was not recommended duo to the unpredictable side effects of them, which may be dangerous for children without sufficient monitor during the long procedure of MRI (18). While in premedication for children before surgeries, the situation was quite different. So the combination of midazolam with S-ketamine was adopted in the current study, which was proved to provide adequate level of sedation for separation, meanwhile significantly reduced the oral dose of midazolam.
Intranasal administration is a noninvasive route without potential side effects and complications such as nerve injury, inadvertent IV or arterial injection, and infection that are associated with intramuscular drug administration. Absorption of intranasal drugs occurs directly into central circulation, bypassing the enterohepatic circulation (19). Intranasal dexmedetomidine is a preferable method for premedication to many pediatric anesthesiologists due to its high bioavailability [81.8% (72.6–92.1%)], easily arousable, lower irritation to buccal mucosa, satisfactory parenteral separation and less incidence of postoperative agitation (20). However, the longer onset time from 25 to 45 min is the shortcoming of dexmedetomidine (21), and so is the midazolam. According to the study of McMillan, the onset time of oral midazolam was reported to be 30–45 min (22). Thus, the intranasal route of S-ketamine was adopted for the purpose of shortening the onset time of sedation in the study. As a result, the onset time was about 10 min shorter in MS group than that in M group, which was close to the report of Khatavkar et al. (14). Although the different of sedative criterion judgements may cause the different onset time, it still showed midazolam combined with S-ketamine could accelerate the onset time by the observation in the level of sedation, or serial changes of HR of the children.
Solo use of S-ketamine or ketamine as a drug of premedication for children was controversial duo to their higher doses. Marhofer et al. demonstrated 1.5 mg/kg S-ketamine for premedication through rectal route showed poor sedative effect and a frequent incidence of side effect as a result (23). Tanaka et al. showed rectally administration of 10 mg/kg ketamine was as effective as 1 mg/kg midazolam for sedation of healthy children, however, the method had the shortcoming of delayed onset and prolonged postoperative sedation (24). The intranasal dose of S-ketamine was much smaller in this study than that of the studies above, and with the combination of oral midazolam, the side effects duo to the high doses were rare.
Since, few studies of intranasal S-ketamine was reported, a low dose of IV 0.25 mg/kg S-ketamine was adopted in two studies (25,26), so this small dose was also chosen for the current study. However, the common point in failed cases of MS group was the children with lower weight because of younger age. And this may remind us that a fixed floor dose or higher intranasal dose of S-ketamine according to the weight could be adopted in the case of 2–3-year-old children, and further research could be done to determine the ED90 dose of intranasal S-ketamine based on the current oral dose of midazolam.
Although a large part of children in MS group and M group were still awaken at the time of separations or during the transportation to OR according to the result of the study, they could still achieve satisfactory separation scores and sedation level. This may be the highlight of the study, which may attribute to the low dose of S-ketamine and midazolam. A satisfactory premedication should first guarantee the safety of children before anesthesia as well as exert little interact with the general anesthetics.
Both midazolam and S-ketamine have synergistic effect with general anesthetics. So the doses of general anesthetics for induction and maintaining should be reduced and some drugs with long half-life period should be avoided in short surgeries. In a study of premedication with nasal S-ketamine and midazolam, the induction and maintaining of general anesthesia was with gases, and no additional opioids or IV sedatives were given (26). In this study, anesthesia was induced with 2 mg/kg propofol, 2 µg/kg fentanyl and 0.6 mg/kg rocuronium and maintained with 5 mg/kg propofol and low concentration of sevoflurane. S-ketamine, midazolam and dexmedetomidine were avoided during the anesthesia. As a result, children in both groups were within normal time at extubating and stay in PACU. But we still found the extubation time and recovery time were relatively longer in M group than those in MS group, and in some extremely short surgeries than those in longer surgeries, which may remind us that the combination of S-ketamine and midazolam in premedication was recommended, while the induction and maintaining of anesthesia should be simplified and the dose should be reduced in extreme short surgeries for children with premedication.
As we know, S-ketamine has both analgesic and sedative effect (22). In the study, the sedative effect of it has been proved, but the percentage of rescue analgesic drug use in PACU was similar between two groups, and the post-operative analgesic effect of S-ketamine was not exhibited. Maybe the dose of intranasal S-ketamine was too low to produce its analgesic effect. Research with larger examples could be done to further observe the analgesic effect of low intranasal dose of S-ketamine.
There were some limitations in the study. First, both oral and intranasal routines of premedication were adopted in the study. Although the combination of both routines of premedication had been used before (27), it was quite complicated for children and nurses in the procedure of premedication. The intention of the combination oral midazolam with intranasal S-ketamine was to improve the onset time. Consequently, only a small part of children refused or failed in intranasal S-ketamine after taking the midazolam syrup and the onset time was shorter than those in M group. Second, the degrees of parents’ anxiety were not measured in the study, and due to the satisfactory separation scores of children in the study, it can be imagined that the level of parents’ anxiety could be controlled. But further research could be carried out in this respect. Third, surgeries with relatively long duration were excluded from the study so that the influences of both premedical drugs to the extubating and post anesthesia recovery could be observed. And the side effects of both drugs for the longer duration of surgery could be observed further.
Conclusions
As for the premedication for preschool children, the ED90 dose of oral midazolam was 0.461 mg/kg (95% CI: 0.425–0.488), and the ED90 dose of oral midazolam was 0.253 mg/kg (95% CI: 0.242–0.278) when combined with intranasal S-ketamine. Oral midazolam with intranasal S-ketamine has the advantage of quick onset, less incidence of behavioral changes and faster recovery.
Acknowledgments
The work was attributed to Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Funding: This work was supported by Foundation of Shanghai Science and Technology Committee (20DZ2306700).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-247
Trial Protocol: Available at https://dx.doi.org/10.21037/tp-21-247
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tp-21-247
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-247). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Research Ethics Committee of Shanghai Child Medical Centre (K2020059-2) and the parents or legal guardians from each participant provided written informed consent for participation in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mehdi I, Parveen S, Choubey S, et al. Comparative Study of Oral Midazolam Syrup and Intranasal Midazolam Spray for Sedative Premedication in Pediatric Surgeries. Anesth Essays Res 2019;13:370-5. [Crossref] [PubMed]
- Manoj M, Satya Prakash MVS, Swaminathan S, et al. Comparison of ease of administration of intranasal midazolam spray and oral midazolam syrup by parents as premedication to children undergoing elective surgery. J Anesth 2017;31:351-7. [Crossref] [PubMed]
- McErlean M, Bartfield JM, Karunakar TA, et al. Midazolam syrup as a premedication to reduce the discomfort associated with pediatric intravenous catheter insertion. J Pediatr 2003;142:429-30. [Crossref] [PubMed]
- Gupta A. Comparison of Oral Triclofos and Oral Midazolam as Premedication in Children undergoing Elective Surgery. Anesth Essays Res 2019;13:366-9. [Crossref] [PubMed]
- Salman S, Tang EKY, Cheung LC, et al. A novel, palatable paediatric oral formulation of midazolam: pharmacokinetics, tolerability, efficacy and safety. Anaesthesia 2018;73:1469-77. [Crossref] [PubMed]
- Impellizzeri P, Vinci E, Gugliandolo MC, et al. Premedication with melatonin vs midazolam: efficacy on anxiety and compliance in paediatric surgical patients. Eur J Pediatr 2017;176:947-53. [Crossref] [PubMed]
- Johnson E, Briskie D, Majewski R, et al. The physiologic and behavioral effects of oral and intranasal midazolam in pediatric dental patients. Pediatr Dent 2010;32:229-38. [PubMed]
- Oriby ME. Comparison of Intranasal Dexmedetomidine and Oral Ketamine Versus Intranasal Midazolam Premedication for Children Undergoing Dental Rehabilitation. Anesth Pain Med 2019;9:e85227 [PubMed]
- Weber F, Wulf H, Gruber M, et al. S-ketamine and s-norketamine plasma concentrations after nasal and i.v. administration in anesthetized children. Paediatr Anaesth 2004;14:983-8. [Crossref] [PubMed]
- Banerjee B, Bose A, Pahari S, et al. A comparative study of paediatric oral premedication: midazolam, ketamine and low dose combination of midazolam and ketamine. J Indian Med Assoc 2011;109:386-8. [PubMed]
- Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007;107:144-52. [Crossref] [PubMed]
- Golparvar M, Saghaei M, Sajedi P, et al. Paradoxical reaction following intravenous midazolam premedication in pediatric patients - a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth 2004;14:924-30. [Crossref] [PubMed]
- Ghai B, Grandhe RP, Kumar A, et al. Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Paediatr Anaesth 2005;15:554-9. [Crossref] [PubMed]
- Khatavkar SS, Bakhshi RG. Comparison of nasal Midazolam with Ketamine versus nasal Midazolam as a premedication in children. Saudi J Anaesth 2014;8:17-21. [Crossref] [PubMed]
- Darlong V, Shende D, Subramanyam MS, et al. Oral ketamine or midazolam or low dose combination for premedication in children. Anaesth Intensive Care 2004;32:246-9. [Crossref] [PubMed]
- Funk W, Jakob W, Riedl T, et al. Oral preanaesthetic medication for children: double-blind randomized study of a combination of midazolam and ketamine vs midazolam or ketamine alone. Br J Anaesth 2000;84:335-40. [Crossref] [PubMed]
- Weber F, Wulf H, el Saeidi G. Premedication with nasal s-ketamine and midazolam provides good conditions for induction of anesthesia in preschool children. Can J Anaesth 2003;50:470-5. [Crossref] [PubMed]
- Schulte-Uentrop L, Goepfert MS. Anaesthesia or sedation for MRI in children. Curr Opin Anaesthesiol 2010;23:513-7. [Crossref] [PubMed]
- Sarkar MA. Drug metabolism in the nasal mucosa. Pharm Res 1992;9:1-9. [Crossref] [PubMed]
- Bhat R, Santhosh MC, Annigeri VM, et al. Comparison of intranasal dexmedetomidine and dexmedetomidine-ketamine for premedication in pediatrics patients: A randomized double-blind study. Anesth Essays Res 2016;10:349-55. [Crossref] [PubMed]
- Yuen VM, Hui TW, Irwin MG, et al. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia 2010;65:922-9. [Crossref] [PubMed]
- McMillan CO, Spahr-Schopfer IA, Sikich N, et al. Premedication of children with oral midazolam. Can J Anaesth 1992;39:545-50. [Crossref] [PubMed]
- Marhofer P, Freitag H, Höchtl A, et al. S(+)-ketamine for rectal premedication in children. Anesth Analg 2001;92:62-5. [Crossref] [PubMed]
- Tanaka M, Sato M, Saito A, et al. Reevaluation of rectal ketamine premedication in children: comparison with rectal midazolam. Anesthesiology 2000;93:1217-24. [Crossref] [PubMed]
- Bornemann-Cimenti H, Wejbora M, Michaeli K, et al. The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol 2016;82:1069-76. [PubMed]
- Trimmel H, Helbok R, Staudinger T, et al. S(+)-ketamine: Current trends in emergency and intensive care medicine. Wien Klin Wochenschr 2018;130:356-66. [Crossref] [PubMed]
- Yuen VM, Hui TW, Irwin MG, et al. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg 2008;106:1715-21. [Crossref] [PubMed]



