
Efficacy of phentolamine combined with ambroxol aerosol inhalation in the treatment of pediatric severe pneumonia and its effect on serum IL-10 and CRP levels
Introduction
Children are susceptible to pathogenic bacteria due to their low immunity and immature respiratory tract, which can trigger pneumonia (1,2). Pediatric pneumonia, a common respiratory disease with acute onset and rapid progression, often occurs in infants and young children due to genetic, environmental, nutritional, and other factors. If infants or children are not effectively treated, the disease can develop into pediatric severe pneumonia (3). Pediatric severe pneumonia is mainly characterized by ventilation dysfunction and fever symptoms (in the early stage), accompanied by hypoxemia, and has a high mortality rate. Therefore, it is important to treat the disease as soon as possible. At present, azithromycin and erythromycin are often used for antibacterial treatment in clinical practice, with unsatisfactory efficacy and obvious complications. Therefore, it is crucial to select proper anti-inflammatory drugs (4-6). Phentolamine is a non-selective α-receptor blocker that can induce lower blood pressure and vasodilation by blocking α1 and α2 receptors in blood vessels, and can enhance myocardial contractility. Specifically, it can improve cardiac function, accelerate vascular expansion, alleviate vascular resistance, and significantly reduce peripheral vascular resistance, thus effectively improving the clinical symptoms and signs of patients with pneumonia. At the same time, the alleviation of clinical symptoms promotes the improvement of inflammatory response to a certain extent, but the effect lasts for a short time. Ambroxol is a common drug to treat pediatric pneumonia by activating pulmonary surfactant and promoting the discharge of retained sputum. It is speculated that the combination of the 2 drugs can have a better therapeutic effect (7,8). At present, few studies have reported the therapeutic effect of the combination of phentolamine and ambroxol. In order to determine their efficacy, 85 cases of children with severe pneumonia diagnosed and treated in our hospital were selected as the research subjects, summarized as follows. We present the following article in accordance with the TREND reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-516/rc).
Methods
General information
Eighty-five children with severe pneumonia treated in our hospital from November 2019 to November 2020 were selected as the research participants, and were divided into the routine group (n=41) and treatment group (n=44) according to odd and even admission numbers, respectively. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013) (9). The study was approved by ethics committee of Yantai Mountain Hospital (No. 20190977) and individual consent for this retrospective analysis was waived.
Inclusion criteria
The inclusion criteria were as follows: (I) diagnostic criteria and clinical symptoms of severe pneumonia, including severe cough, expectoration, chest pain, dyspnea, wheezing, cyanosis, diffuse fixed rales in the lungs (mainly small vesicular sounds), and signs of diffuse infection in the lungs confirmed by chest CT; (II) no congenital heart disease; and (III) age 3–13 years.
Exclusion criteria
The exclusion criteria were as follows: (I) immune deficiency; (II) pulmonary tuberculosis; (III) liver and kidney dysfunction; (IV) unable to cooperate; and (V) allergic to the drugs used in the research.
Treatments
Children in the routine group received clinical routine treatment, including cough relief (e.g., expectorant), defervescence, anti-infection treatment, maintenance of acid-base balance, and other symptomatic treatment measures (10,11).
Children in the treatment group were treated with phentolamine combined with Ambroxol aerosol inhalation. The children received 0.5 mg/kg phentolamine (Bikang Pharmaceutical Xinyi Group Holding) mixed with 50 mL of 5% glucose injection 1–2 times each day. The children also received aerosol inhalation of Ambroxol hydrochloride (Shanghai Boehringer Ingelheim Pharmaceutical), 15 mg 1–2 times per day. Both groups were treated for 7 days to observe the therapeutic effect.
Observation indexes
The duration of clinical symptoms and hospitalization time were recorded and compared between the 2 groups. Clinical symptoms included cough, fever, abnormal lung noise, and lung shadow.
The curative effect of both groups after treatment was evaluated according to the following criteria. If the children’s body temperature after 3 days of treatment returned to normal, and symptoms such as cough and expectoration disappeared, the children were cured. If the body temperature after 3 days of treatment returned to normal, and symptoms, such as cough and expectoration, were relieved, the treatment was markedly effective. If the body temperature after 3 days of treatment gradually returned to normal, and symptoms, such as cough and expectoration, were partially relieved, the treatment was effective. If the body temperature did not drop and clinical symptoms were not relieved, the treatment was ineffective. The total effective rate = (number of cured cases + number of markedly effective cases + number of effective cases) / total number of cases × 100%.
Pulmonary function indexes
A spirometer (RSFJ1000; Chengdu Risheng Electric) was used to detect changes in forced vital capacity (FVC) and peak expiratory flow rate (PEF) levels in both groups before and after treatment.
A total of 3 mL fasting venous blood was collected from children in both groups before treatment (T0), 2 days after treatment (T1), 5 days after treatment (T2), and 7 days after treatment (T3), and serum was collected after centrifugation. Enzyme-linked immunosorbent assay was used to determine interleukin-10 (IL-10) and C-reactive protein (CRP) levels in the serum samples. The kits were purchased from Shenzhen Ziker Biological Technology and used according to the manufacturer’s instructions.
The incidence of clinical adverse reactions was recorded and compared between the 2 groups.
Statistical methods
All experimental data were statistically analyzed and processed using SPSS version 21.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Count data were analyzed using χ2-test and expressed as n (%). Measurement data were analyzed using t-test and expressed as . The difference was statistically significant when P<0.05.
Results
Comparison of general clinical data between the 2 groups
There were no significant differences in sex ratio, average age, average course of disease, average body temperature, dyspnea, loss of appetite, and residence of the patients between the 2 groups (P>0.05), indicating comparability, as shown in Table 1.
Table 1
| Items | Treatment group (n=41) | Routine group (n=44) | χ2/t | P value |
|---|---|---|---|---|
| Sex, n (%) | 0.021 | 0.886 | ||
| Male | 23 (56.10) | 24 (54.55) | ||
| Female | 18 (43.90) | 20 (45.45) | ||
| Age (years) | 7.42±2.31 | 7.37±2.36 | 0.099 | 0.922 |
| Course of disease (days) | 8.24±1.63 | 8.29±1.58 | 0.144 | 0.886 |
| Body temperature (°C) | 38.62±0.53 | 38.67±0.47 | 0.461 | 0.646 |
| Dyspnea, n (%) | 0.201 | 0.654 | ||
| Yes | 28 (68.29) | 32 (72.73) | ||
| No | 13 (31.71) | 12 (27.27) | ||
| Loss of appetite, n (%) | 0.031 | 0.859 | ||
| Yes | 25 (60.98) | 26 (59.09) | ||
| No | 16 (39.02) | 18 (40.91) | ||
| Residence, n (%) | 0.572 | 0.450 | ||
| Urban area | 22 (53.66) | 20 (45.45) | ||
| Rural area | 19 (46.34) | 24 (54.55) |
Comparison of duration of clinical symptoms and hospitalization time between the 2 groups
The duration of cough, fever, abnormal lung sound, and lung shadow, and hospitalization time in the treatment group were significantly shorter than those in the routine group (P<0.001), as shown in Table 2.
Table 2
| Groups | n | Duration (days) | Hospitalization time | |||
|---|---|---|---|---|---|---|
| Cough | Fever | Abnormal lung sound | Lung shadow | |||
| Treatment group | 41 | 5.23±1.42 | 4.53±1.38 | 9.62±1.36 | 8.41±2.31 | 7.24±3.15 |
| Routine group | 44 | 6.72±1.25 | 5.63±1.58 | 11.25±1.47 | 11.68±2.58 | 12.96±4.03 |
| t | 5.143 | 3.408 | 5.295 | 6.140 | 7.254 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
Comparison of clinical efficacy between the 2 groups
The total clinical effective rate in the treatment group was significantly higher than that in the routine group (P<0.05), as shown in Table 3.
Table 3
| Groups | n | Cured, n (%) | Markedly effective, n (%) | Effective, n (%) | Ineffective, n (%) | Total effective rate |
|---|---|---|---|---|---|---|
| Treatment group | 41 | 14 (34.15) | 15 (36.59) | 10 (24.39) | 2 (4.88) | 95.12% (39/41) |
| Routine group | 44 | 9 (20.45) | 14 (31.82) | 12 (27.27) | 9 (20.45) | 79.55% (35/44) |
| χ2 | 4.571 | |||||
| P value | 0.033 |
Comparison of pulmonary function indexes before and after treatment between the 2 groups
Both groups had higher FVC and PEF levels following treatment (P<0.05), and these levels were higher in the treatment group compared with the routine group after treatment (P<0.05), as shown in Figures 1,2.
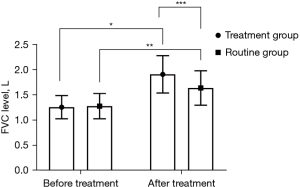
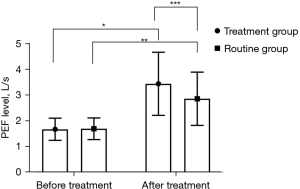
Comparison of serum IL-10 and CRP level changes between the 2 groups at different time points
Serum IL-10 and CRP levels at T1, T2, and T3 in the treatment group were significantly lower than those in the routine group (P<0.001), as shown in Figures 3,4.
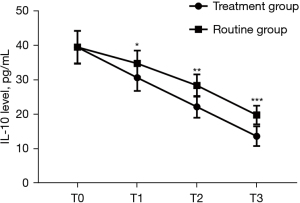
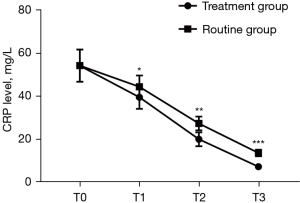
Comparison of therapeutic safety between the 2 groups
The total incidence of adverse reactions in the treatment group was significantly lower than that in the routine group (P<0.05), as shown in Table 4.
Table 4
| Groups | n | Gastrointestinal reaction, n (%) | Phlebitis, n (%) | Rash, n (%) | Abnormal liver function, n (%) | Total incidence |
|---|---|---|---|---|---|---|
| Treatment group | 41 | 2 (4.88) | 0 (0.00) | 1 (2.44) | 1 (2.44) | 9.76% (37/41) |
| Routine group | 44 | 3 (6.82) | 4 (9.09) | 3 (6.82) | 2 (4.55) | 27.27% (32/44) |
| χ2 | 4.262 | |||||
| P value | 0.039 |
Discussion
A study (12) has confirmed that pneumonia is a lung inflammatory response caused by environmental or viral factors. Early clinical symptoms of pediatric pneumonia, such as mild cough, are not obvious, and are often ignored by parents, and can result in severe pneumonia (13-15). Clinically, pneumonia is divided into prolonged pneumonia, acute pneumonia, and chronic pneumonia, according to the course of the disease, while severe pneumonia is a more serious stage in the development of pneumonia. Children are prone to infection because of softer tracheal walls and narrower bronchi compared with adults, and are unable to resist the invasion of pathogens (16,17). If pathogens continue to invade other tissues, swelling of airway mucosa will occur, resulting in restricted ventilation function, and eventually severe pneumonia. In addition to the damage to the respiratory system of children, severe pneumonia also causes neurological, digestive, circulatory, and other dysfunctions, as well as cerebral edema, mental confusion, irregular respiratory rhythm, and even respiratory arrest (18-20).
Pneumonia is type of pulmonary edema, with congestion caused by a variety of pathogens. Its clinical symptoms include fever, expectoration, and dyspnea, as well as the shadow of inflammatory infiltration on lung X-ray (21,22). In the present study, both groups of children received basic treatment, including anti-infection, expectorant, and defervescence, while the treatment group additionally received phentolamine combined with Ambroxol aerosol inhalation. Phentolamine can effectively relieve spasm of bronchial smooth muscle in children, reduce airway resistance, improve ventilation function, and alleviate clinical symptoms, such as cough and lung moist rales. As a new mucolytic drug, ambroxol can effectively break the viscous polysaccharide fibers, weaken the adhesion of mucus, reduce the alveolar surface tension, affect the activity of respiratory enzymes in epithelial villi, and improve the mucociliary transport of respiratory mucosa. Phentolamine combined with ambroxol can play a good role in the dilation of bronchial smooth muscle, reduce venous pressure, improve pulmonary circulation, and enhance the curative effect by reducing myocardial oxygen consumption and reducing cardiac load. With a synergistic effect, their combination is more effective than the single drug treatment. As shown in Table 2, X-ray and clinical diagnosis of children showed that the duration of cough, fever, abnormal lung sound, and lung shadow in the treatment group was significantly shorter than those in the routine group (P<0.001), indicating that Ambroxol aerosol inhalation combined with phentolamine can effectively shorten the regression time of clinical symptoms in children. In addition, a previously published study (23) confirmed that severe pneumonia can cause disorders in body immune response in children, activate and release inflammatory factors into the blood, and induce respiratory tract inflammation. Inflammatory response is involved in the occurrence and development of the disease. After expansion, inflammatory response occurs throughout the body and in the lungs, with a large number of inflammatory factors in the alveoli. CRP and IL-10 are important inflammatory factors that can reflect the severity of infection in the body. CRP reduces inflammatory response by activating complement phagocytes and enhancing their role. Therefore, CRP levels are highly expressed in children with severe pneumonia. Serum IL-10 is a multifunctional cytokine that regulates and mediates the function of immune cells, and affects the occurrence of immune damage and inflammation. Therefore, serum IL-10 levels are highly expressed in children with severe pneumonia (24). After treatment, serum IL-10 and CRP levels in both groups decreased, and serum IL-10 and CRP levels at T1, T2, and T3 in the treatment group were significantly lower than those in the routine group (P<0.05). In their study, Nasser et al. found that, after children with severe pneumonia mycoplasma pneumonia were treated with azithromycin combined with phentolamine, CRP was 16.31±4.28 mg/L, which was significantly lower than 56.72±6.37 mg/L before treatment, suggesting that the combination of drugs can effectively inhibit bronchospasm, increase vascular permeability, reduce airway inflammation, and have a significant anti-inflammatory effect in children (25). In addition, the incidence of clinical adverse reactions in the treatment group was significantly lower than that in the routine group, demonstrating that phentolamine combined with Ambroxol aerosol inhalation not only improves the therapeutic effect on children with severe pneumonia but also has high safety.
In conclusion, phentolamine combined with Ambroxol aerosol inhalation not only effectively relieves the clinical discomfort symptoms of children with severe pneumonia but also reduces the inflammatory factor levels and controls disease development with high safety.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-516/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-516/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-516/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Yantai Mountain Hospital (No. 20190977) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bardsley EN, Neely OC, Paterson DJ. Angiotensin peptide synthesis and cyclic nucleotide modulation in sympathetic stellate ganglia. J Mol Cell Cardiol 2020;138:234-43. [Crossref] [PubMed]
- Vinnakota DN, Kamatham R. Safety profile of phentolamine mesylate as reversal agent of pulpal and soft tissue dental anesthesia: a systematic review and meta-analysis. Quintessence Int 2019;50:568-75. [PubMed]
- Sartori MR, Kohl ZF, Taylor EW, et al. Blood flow distribution in embryonic common snapping turtles Chelydra serpentina (Reptilia; Chelonia) during acute hypoxia and α-adrenergic regulation. Comp Biochem Physiol A Mol Integr Physiol 2019;238:110575. [Crossref] [PubMed]
- Dou Y, Luo J, Yu J, et al. Cholinergic system is involved in the therapeutic effect of madecassoside on collagen-induced arthritis in rats. Int Immunopharmacol 2019;75:105813. [Crossref] [PubMed]
- Peng L, Wang Y, Zhao L, et al. Severe pneumonia in Chinese patients with systemic lupus erythematosus. Lupus 2020;29:735-42. [Crossref] [PubMed]
- Baek MS, Park S, Choi JH, et al. Mortality and Prognostic Prediction in Very Elderly Patients With Severe Pneumonia. J Intensive Care Med 2020;35:1405-10. [Crossref] [PubMed]
- Li Q, Liang F, Sang L, et al. Pharmacokinetics of and maintenance dose recommendations for vancomycin in severe pneumonia patients undergoing continuous venovenous hemofiltration with the combination of predilution and postdilution. Eur J Clin Pharmacol 2020;76:211-7. [Crossref] [PubMed]
- Dai J, Xiong Y, Li H, et al. Corticosteroid treatment in severe COVID-19 pneumonia: two cases and literature review. Clin Rheumatol 2020;39:2031-7. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Uddin Chowdhury MR, Akter KS, Alam MZ, et al. COVID-19 Disease Complicated with Severe Pneumonia in a Patient with End Stage Renal Disease (ESRD): A Case Report from Bangladesh. Advances in Infectious Diseases 2020;10:101-9. [Crossref]
- Cvetanovska M, Milenovic Z, Grozdanovski K, et al. The Impact of Pneumonia on the Course and Outcome in Patients with Seasonal Influzenza. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2020;41:63-70. [Crossref] [PubMed]
- Guo X, Yang D, Liu R, et al. Detecting influenza and emerging avian influenza virus by influenza and pneumonia surveillance systems in a large city in China, 2005 to 2016. BMC Infect Dis 2019;19:825. [Crossref] [PubMed]
- Gavrieli H, Dagan R, Givon-Lavi N, et al. Unique Features of Hospitalized Children with Alveolar Pneumonia Suggest Frequent Viral-Bacterial Coinfections. Pediatr Infect Dis J 2020;39:586-90. [Crossref] [PubMed]
- Zhang C, Rong HM, Li T, et al. PD-1 Deficiency Promotes Macrophage Activation and T-Helper Cell Type 1/T-Helper Cell Type 17 Response in Pneumocystis Pneumonia. Am J Respir Cell Mol Biol 2020;62:767-82. [Crossref] [PubMed]
- Arnold FW, Reyes Vega AM, Salunkhe V, et al. Older Adults Hospitalized for Pneumonia in the United States: Incidence, Epidemiology, and Outcomes. J Am Geriatr Soc 2020;68:1007-14. [Crossref] [PubMed]
- Martens T, Saini R, Crook R, et al. Deep hypothermic extracorporeal membrane oxygenation cannula exchange in a child with necrotic pneumonia. Perfusion 2020;35:169-71. [Crossref] [PubMed]
- Craven TH, Wojcik G, McCoubrey J, et al. Ventilator-associated pneumonia surveillance using two methods. J Hosp Infect 2020;104:522-8. [Crossref] [PubMed]
- Chumbita M, Cillóniz C, Puerta-Alcalde P, et al. Can Artificial Intelligence Improve the Management of Pneumonia. J Clin Med 2020;9:248. [Crossref] [PubMed]
- Barreto JN, Thompson CA, Wieruszewski PM, et al. Incidence, clinical presentation, and outcomes of Pneumocystis pneumonia when utilizing Polymerase Chain Reaction-based diagnosis in patients with Hodgkin lymphoma. Leuk Lymphoma 2020;61:2622-9. [Crossref] [PubMed]
- Bernshteyn M, Kumar PA, Joshi S. Kocuria kristinae pneumonia and bacteremia. Proc (Bayl Univ Med Cent) 2020;33:608-9. [Crossref] [PubMed]
- Nakamura M, Nagamine T. Eosinophilic pneumonia during treatment with clozapine: reports from a retrospective case series. Int Clin Psychopharmacol 2020;35:285-91. [Crossref] [PubMed]
- Rivero-Calle I, Pardo Seco J, Raguindin PF, et al. Routine infant vaccination of pneumococcal conjugate vaccines has decreased pneumonia across all age groups in Northern Spain. Hum Vaccin Immunother 2020;16:1446-53. [Crossref] [PubMed]
- Morrison NR, Johnson SM, Hocker AD, et al. Time and dose-dependent impairment of neonatal respiratory motor activity after systemic inflammation. Respir Physiol Neurobiol 2020;272:103314. [Crossref] [PubMed]
- Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant 2020;20:1859-63. [Crossref] [PubMed]
- Nasser H, Ivanics T, Shakaroun D, et al. Severe phlebitis-like abnormal reaction following great saphenous vein cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord 2019;7:578-82. [Crossref] [PubMed]


