
A novel variant site of Alstrom syndrome in a Chinese child: a case report
Introduction
Alstrom syndrome (ALMS, OMIM #203800) is a rare recessive heredity disease caused by variants of the ALMS1 gene. Since it was first pronounced in 1959, no extra than 1,000 cases have been reported (1). ALMS has an estimated incidence of 1 case per 1,000,000 live births (2). ALMS is an uncommon disease, which critically influences their existence great and survival rates. The life span of patients with ALMS rarely exceeds 40 years (3).
ALMS is a multisystem, innovative sickness characterised by childhood obesity, hypertriglyceridemia, insulin resistance, type 2 diabetes, blindness and deafness (2,3). Clinical symptoms of patients with ALMS first appear in infancy with great variability in age of onset and severity (2). In addition, sufferers with ALMS may also go through from liver dysfunction, liver fibrosis, pulmonary manifestations, hypothyroidism, and kidney dysfunction, and tissue fibrosis can show up in nearly all organs (2,3). The rarity and complexity of this syndrome, and lack of expertise often lead to misdiagnosed.
Routine evaluations of vision, hearing, weight, height, and body mass index (BMI) are needed. Blood biochemicals such as transaminase, lipid profile, blood urine nitrogen (BUN), creatinine (Cr), cystatin-C, and uric acid may also be abnormal. Most sufferers present with insulin resistance, and their c-peptide, glucose, and hemoglobin A1C (HbA1C) are additionally abnormal. In addition, urinalysis, thyroid function, renal and bladder ultrasound examinations, and heart evaluations including echocardiography and electrocardiogram (EKG) are additionally essential to assess.
By now, almost 300 pathogenic variants of ALMS have been recognized (2), however, some variant sites are still unclear. Therefore, extra researches are needed to discover the variant types and pathophysiological procedures in-depth to apprehend the onset and development of ALMS, which may lead to the development of reachable treatment strategies for ALMS patients. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-535/rc).
Case presentation
The proband was a 14-year-old boy with a 2-month records of sallow complexion. Ultrasound of liver and spleen of neighborhood hospital suggests the opportunity of Wilson’s disease. The patient attended our hospital for further diagnosis and therapy on 02-Mar-2021. According to the clinical records furnished by the family, he was once born with retinal dystrophy and was later found amblyopia when he was 2 years old. His parents and sister were normal without phenotype. His grandpa was lately observed with abnormal blood glucose.
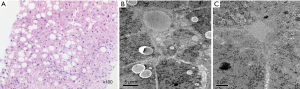
Physical examination showed his weight was 60.3 kg (50th–75th percentile), height was 159 cm (10th–25th percentile), and BMI was 23.5. These results showed that he was overweight. The test of random blood glucose was 11.1 mmol/L, fasting blood glucose was 8.2 mmol/L, insulin was 85.5 μIU/mL, glycated hemoglobin was 9.1%, and C-peptide was 9.33 ng/mL. Diabetic antibodies were negative. These results indicated type 2 diabetes. Triglyceride was 7 mmol/L, and total cholesterol, high-density lipoprotein, low-density lipoprotein, cortisol and adrenocorticotropic hormone (ACTH) were normal. The child presented with nystagmus and blurred vision in both eyes. Visual acuity examination showed visual acuity was 0.1 in the left eye and 0.2 in the right eye. Ophthalmoscope examination showed blurred fundus in both eyes and thickening of optic nerve fiber layers above and below the temporal. Physical examination showed that the lower margin of the liver was about 2 cm below the right costal midline, and the lower margin of the spleen was about 5 cm below the left costal midline. And liver and spleen were medium texture. The liver function test showed that the alanine aminotransferase (ALT) was 91 U/L and the aspartate aminotransferase (AST) was 75 U/L. Ultrasound of liver and spleen showed enhanced echo and thickening of liver parenchyma and splenomegaly. The median and interquartile range (IQR) value of controlled attenuation parameter (CAP, dB/m) were 229 and 37 respectively, which were measured by transient elastography. These results showed that steatosis in liver was no more than 11%. The median and IQR value of liver stiffness measurement (LSM, kPa) were 26.6 (F4) and 2.0, which were measured by transient elastography too. These results indicated that his liver was cirrhotic probably. Hematoxylin-eosin staining of liver tissue was shown by light microscopy (Figure 1A) demonstrating the existence of hepatic lobule structure, mild hydrodegeneration and steatosis of hepatocytes, punctate necrosis, and scattered chronic inflammatory cell infiltration in portal area. The result of electron microscope of liver tissue (Figure 1B,1C) also showed abnormalities. Hepatocytes were mildly swollen, cytoplasmic rough endoplasmic reticulum was reduced, and the smooth endoplasmic reticulum was slightly hyperplastic and dilated. Small lipid droplets were slightly increased with a few cholestatic pigment granules were found in the cytoplasm of some hepatocytes. The interfacial space of hepatocytes was slightly widened while the cholestatic bile duct was slightly dilated. Hepatic stellate cells were easily observed in the Disse lumen, and kupffer cells were observed in hepatic sinusoid. Urine examination showed that urine protein was positive. Urinary albumin (UALB)/Cr, urine microalbumin and urine β2 microglobulin were 731.7 µg/mg, 227.3 mg/L and 5.63 mg/L, respectively. Serum urea nitrogen, serum Cr and serum uric acid were normal. Renal ultrasound showed enhanced echo in the medulla of both kidneys. Hearing, intelligence and blood pressure were normal. Electrocardiograph, echocardiography and chest radiography showed no abnormalities. These results showed that the heart and hearing are likely not affected, but monitoring is still needed.
ALMS is suspected through his complex and varied clinical manifestations. To test this possibility, next generation sequencing of whole exon sequencing was performed on the child and his parents (Table 1). Compound heterozygous pathogenic variants in ALMS1 gene were found in the child. One of his pathogenic variant sites was c.8158C>T, which was from his father and had been reported previously (4) (Figure 2A). The other site c.3575C>A was from his mother (Figure 2B), which had not been reported before. According to the clinical manifestations, laboratory examination results, and compound heterozygous variant in ALMS1 gene, the disease of the child was confirmed as ALMS. All treatments for the child were symptomatic, such as blood sugar controlling and liver protecting. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient/legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The case report has been approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-IRB20210866).
Table 1
| Gene | Chromosomal location | Variant | Type | Genetic model | Source of variation | ACMG evidence item | ACMG variation classification |
|---|---|---|---|---|---|---|---|
| ALMS1 | chr2:73717247 | NM_015120; exon10:c.8158C>T (p.R2720X) | Het | AR | Father | PVS1 + PM3_Strong + PM2 | Pathogenic |
| ALMS1 | chr2:73677232 | NM_015120; exon8:c.3575C>A (p.S1192X) | Het | AR | Mother | PVS1 + PM2 + PM3 | Pathogenic |
Het, heterozygous mutation; AR, autosomal recessive inheritance; ACMG, The American College of Medical Genetics and Genomics.

Discussion
ALMS is a rare autosomal recessive genetic sickness precipitated via ALMS1 gene variants, first pronounced by Alstrom et al. in 1959 (5). ALMS1 gene is located on chromosome 2 (region 2p13.1) (6,7) and contains 23 coding exons that involve 224 kilobase (kb) (8,9). ALMS1 protein is broadly expressed in mammalian cells, along with retina, ear, pancreas, liver and kidney, often positioned in the centrosomes and matrix of primary cilia, and is associated to the formation, maintenance and functional expression of primary cilia (10,11). ALMS1 protein is also involved in intracellular body transport, energy/metabolic balance, cell differentiation, cell cycle, ciliary signaling pathway, etc. (12). ALMS is a multisystem, progressive disease characterised by visual disturbance, hearing impairment, cardiomyopathy, childhood obesity, excessive insulin resistance, accelerated non-alcoholic fatty liver disorder (NAFLD), renal dysfunction, respiratory disease, endocrine, and urologic problems (2,6).
Visual disturbance is one of the main clinical manifestations of ALMS, which is usually the first noticeable manifestation, appearing during the first year of life. Clinical symptoms evolve with age and can lead to blindness before the age of 20 (2). In this paper, the child was observed with retinal dystrophy rapidly after birth, and was found amblyopia when he was 2 years old. Hearing impairment is the second most common manifestation of ALMS and is characterised by progressive bilateral sensorineural hearing loss. The proportion of hearing loss is 70% before the age of 10 years, and the lifetime risk of deafness is 100% (2). Although the child in this case had not yet developed hearing loss and deafness, there is still a high risk of subsequent deafness.
Metabolic disorders are also a major feature of ALMS, which begin in childhood and can lead to rapid weight gain, insulin resistance and type 2 diabetes (13). This patient also had these manifestations. Obesity is one of the main characteristics of ALMS, and most of children with ALMS can develop obesity (14). The BMI of this patient was 23.5 and he used to be overweight. If dietary control and other measures have been no longer taken, there would be an excessive opportunity of obesity in the future. Most ALMS sufferers have reasonable to severe hypertriglyceridemia, with normal total cholesterol and low high-density lipoprotein (HDL) cholesterol (15). The child shows significantly high triglyceride, low HDL and normal total cholesterol and LDL, which are consistent with the syndrome.
ALMS patients show a high incidence of NAFLD and are prone to non-alcoholic steatohepatitis and liver fibrosis (2,16). Transaminase is usually elevated during early childhood, and liver disease in some patients will progress to cirrhosis or liver failure between the ages of 20 and 30 (2,16). In addition, ALMS patients may also develop portal hypertension with splenomegaly, esophageal varices, ascites and hepatic encephalopathy (2,16). In this case, ALT and AST were increased. Ultrasound of liver and spleen indicated hepatosplenomegaly. The test of liver hardness showed median E value was 26.6 (F4), suggesting the possibility of liver cirrhosis. However, the liver biopsy by light microscopy and electron microscopy didn’t show cirrhosis, and it was still considered to be the stage of liver fibrosis. Besides, there was no other manifestations of portal hypertension except for splenomegaly, and they should be monitored in the future.
Chronic kidney disease is also common in patients with ALMS; however, it progresses slowly. The onset time can be from middle childhood to adulthood, and end-stage renal disease can occur as early as middle to late adolescence (17). In this case, the child showed signs of proteinuria, with elevated urinary microalbumin and β2 microglobulin, however, serum urea nitrogen, serum Cr and serum uric acid were normal. Renal ultrasound showed enhanced echo in the medulla of both kidneys. These results suggested that his kidney was involved, however, it was not yet severe. These results are consistent with the characteristics of kidney damage in patients with ALMS. Other clinical manifestations, such as congestive heart failure, cardiomyopathy, hypothyroidism, growth hormone deficiency, male hypogonadism, and recurrent respiratory tract infection, are also common in ALMS patients (2). In this case, we had screened for these manifestations and none of them were found.
The diagnosis of ALMS is mainly based on clinical symptoms. If symptoms extended and worsen with age, the diagnosis of ALMS is supported extra. To confirm ALMS requires genetic tests, because there are no biochemical, histological, or imaging tests. The detection of homozygous variants or compound heterozygous variants of ALMS1 is the gold standard for the diagnosis of ALMS. So far, nearly 300 pathogenic variants have been recognized in ALMS, and they were mainly targeted on exons 8 (6.1 kB), 10 (1.9 kB), and 16 (1.2 kB), which accounted for 51%, 16%, and 17% of the total variants in ALMS1, respectively, and were regarded as hot spot variants (9,18). In this case, ALMS was confirmed by whole exon sequencing after clinical diagnosis. The whole exon sequencing showed that the child had compound heterozygous pathogenic variants of ALMS1 gene, and one of the variant sites was c.8158C>T, which was from his father and had been reported (4). The other variant site c.3575C>A was from his mother, which had not been reported before, to the great of our knowledge. Both of the pathogenic variants result in nonsense variants in amino acids, which makes the synthesis of the peptide chain terminated in advance and the formation of an incomplete polypeptide chain. The new variant site c.3575C>A is the first time to be reported and extends the genetic variant spectrum of ALMS. It is necessary to take a whole exon sequencing for the early diagnosis ALMS, especially for patients with clinical manifestations of this disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-535/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-535/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient/legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The case report has been approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-IRB20210866).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aslam MZ, O'Meachair A, O'Donnell B. Anesthetic Considerations in Alström Syndrome: A Case Report. A A Pract 2021;15:e01503. [Crossref] [PubMed]
- Tahani N, Maffei P, Dollfus H, et al. Consensus clinical management guidelines for Alström syndrome. Orphanet J Rare Dis 2020;15:253. [Crossref] [PubMed]
- Marshall JD, Beck S, Maffei P, et al. Alström syndrome. Eur J Hum Genet 2007;15:1193-202. [Crossref] [PubMed]
- Ozgül RK, Satman I, Collin GB, et al. Molecular analysis and long-term clinical evaluation of three siblings with Alström syndrome. Clin Genet 2007;72:351-6. [Crossref] [PubMed]
- ALSTROM CH. Retinal degeneration combined with obesity, diabetes mellitus and neurogenous deafness: a specific syndrome (not hitherto described) distinct from the Laurence-Moon-Bardet-Biedl syndrome: a clinical, endocrinological and genetic examination based on a large pedigree. Acta Psychiatr Neurol Scand Suppl 1959;129:1-35. [PubMed]
- Collin GB, Marshall JD, Cardon LR, et al. Homozygosity mapping at Alström syndrome to chromosome 2p. Hum Mol Genet 1997;6:213-9. [Crossref] [PubMed]
- Collin GB, Marshall JD, Naggert JK, et al. TGFA: exon-intron structure and evaluation as a candidate gene for Alström syndrome. Clin Genet 1999;55:61-2. [Crossref] [PubMed]
- Hearn T, Renforth GL, Spalluto C, et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alström syndrome. Nat Genet 2002;31:79-83. [Crossref] [PubMed]
- Dassie F, Favaretto F, Bettini S, et al. Alström syndrome: an ultra-rare monogenic disorder as a model for insulin resistance, type 2 diabetes mellitus and obesity. Endocrine 2021;71:618-25. [Crossref] [PubMed]
- Jagger D, Collin G, Kelly J, et al. Alström Syndrome protein ALMS1 localizes to basal bodies of cochlear hair cells and regulates cilium-dependent planar cell polarity. Hum Mol Genet 2011;20:466-81. [Crossref] [PubMed]
- Hearn T. ALMS1 and Alström syndrome: a recessive form of metabolic, neurosensory and cardiac deficits. J Mol Med (Berl) 2019;97:1-17. [Crossref] [PubMed]
- Álvarez-Satta M, Castro-Sánchez S, Valverde D. Alström syndrome: current perspectives. Appl Clin Genet 2015;8:171-9. [PubMed]
- Girard D, Petrovsky N. Alström syndrome: insights into the pathogenesis of metabolic disorders. Nat Rev Endocrinol 2011;7:77-88. [Crossref] [PubMed]
- Engle SE, Bansal R, Antonellis PJ, et al. Cilia signaling and obesity. Semin Cell Dev Biol 2021;110:43-50. [Crossref] [PubMed]
- Paisey RB, Carey CM, Bower L, et al. Hypertriglyceridaemia in Alström's syndrome: causes and associations in 37 cases. Clin Endocrinol (Oxf) 2004;60:228-31. [Crossref] [PubMed]
- Bettini S, Bombonato G, Dassie F, et al. Liver Fibrosis and Steatosis in Alström Syndrome: A Genetic Model for Metabolic Syndrome. Diagnostics (Basel) 2021;11:797. [Crossref] [PubMed]
- Baig S, Paisey R, Dawson C, et al. Defining renal phenotype in Alström syndrome. Nephrol Dial Transplant 2020;35:994-1001. [Crossref] [PubMed]
- Marshall JD, Muller J, Collin GB, et al. Alström Syndrome: Mutation Spectrum of ALMS1. Hum Mutat 2015;36:660-8. [Crossref] [PubMed]


