
Cardiac autonomic modulation in children with severe liver disease, before and after liver transplantation
Introduction
Liver cirrhosis is a common outcome of several hepatic diseases. It is characterized by diffuse fibrosis of the liver, leading to liver failure and portal hypertension (1-3). In children, its main etiologies are biliary atresia, ductopenic syndromes, and tyrosinemia.
In the past decades, several studies have shown repercussions of liver cirrhosis in many organs and systems, such as the urinary tract (4,5), respiratory (6,7), and cardiovascular systems (8,9). Some studies in adult patients have shown that cirrhosis also affects the autonomic nervous system (ANS). Autonomic dysfunction (AD) can be found in up to 60% of patients with cirrhosis (10). AD may represent a direct consequence of a hyperdynamic circulation associated with cirrhotic portal hypertension.
Hyperdynamic circulation may lead to AD through the following mechanisms: (I) increased circulating vasodilators; (II) increased plasma angiotensin‐2 levels; (III) imbalance between sympathetic and parasympathetic functions with predominant vagal dysfunction; (IV) reduced cardiac and peripheral beta‐adrenergic receptors density and impaired reactivity to vasoconstrictor stimuli (10). Liver dysfunction and portal‐systemic shunts increase the level of vasodilators, leading to reduced peripheral vascular responsiveness to vasoconstrictors (11). Although usually asymptomatic (12), AD may correlate with increased mortality and morbidity before, during and after liver transplantation (LT) (13,14).
One of the first subclinical manifestations of AD can be changes in heart rate variability (HRV) (15), a measure that describes oscillations in RR intervals (the distance between two R waves) of consecutive heartbeats, suggesting involvement of the ANS (16,17). The analysis of HRV provides an indirect measure of parasympathetic function. A reduced HRV has been previously confirmed as an independent factor for cardiac ischemic and arrhythmogenic events in high‐risk patients (18) (for example, those with coronary artery disease). Compared to cardiovascular autonomic tests, HRV analysis has shown greater sensitivity.
A mini-review conducted by Amaral and collaborators on HRV and non-alcoholic cirrhosis found a relationship between autonomic responses and cirrhosis even in the earliest stages; concluding that the study of HRV can be used as a complementary method for diagnosis and prognosis of non-alcoholic cirrhosis (19).
Presently, the only effective treatment for liver cirrhosis in children and adults is LT. Among the positive effects of this approach, a regression of AD was found in 63–70% of adult patients in the presence of a normally functioning graft (20,21). However, it is not known if AD in children with severe liver disease presents the same pattern of response as in adults, since the course of AD after LT has never been investigated in children. The objective of the present study was to compare the cardiac autonomic modulation in children with severe chronic liver disease before and after LT, using HRV analysis. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-273/rc).
Methods
This observational study was carried out at the Instituto da Criança, Medicine School of University of São Paulo, from July 2015 to March 2019.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethical committee of the Medicine School of the University of São Paulo (No.: 1.113.629) and informed consent was signed by the patients’ parents or legal guardians who were informed about the procedures and objectives of the study.
Pediatric patients with liver cirrhosis of both sexes, ages 6 months to 10 years old, who were enrolled on the Ministry of Health’s liver transplant waiting list and occupied the top ten positions, were included.
Exclusion criteria included patients with cardiopulmonary disorders not due to cirrhosis, as well as neurological disorders, or any other impediment for the patient to perform the procedures.
Information from medical records was used to collect data and analyze inclusion and exclusion criteria. Diagnostic data, history of the disease, age, weight, height, medications used, and placement in the pediatric end-stage liver disease (PELD) were obtained from medical records.
Following the Institute’s protocol, children with severe liver disease were registered on the waiting list for transplant from a cadaveric donor. Parents were also informed about the possible use of an organ from a living donor; radiological and laboratory exams were then performed to assess this alternative. If a candidate was considered healthy and eligible, we proceeded with liver transplant from the living donor.
The numerical value of the PELD estimates prognosis for pediatric patients (under 12 years old) with liver disease and is used to prioritize patients in the transplant queue. The higher the calculated index, the more critical the patient’s condition. For the calculation of this index, the rates of bilirubin, INR (coagulation factor), and albumin were considered. Also considered were the patient’s age and whether the patient had a growth deficit. To obtain the PELD the following formula is applied. PELD =10× [0.480 × Loge (bilirubin mg/dL) +1.857× Loge (INR) −0.687× Loge (albumin mg/dL) +0.436 if the patient is up to 24 months old, +0.667 if the patient has a growth deficit <2]. In this study, children who were among the top ten in the queue for transplant (PELD score) were evaluated (22).
After the initial identification, the Polar RS800CX heart rate receiver strap was positioned on the volunteers’ chest, in the region of the distal third of the sternum with the capture strap.
Data collection was carried out in a room without a constant flow of people to avoid distractions and unnecessary noise. The children were positioned seated, younger children in the lap of a guardian for greater comfort during the collection procedure. The older children were sitting in chairs. During the collection, conversation was avoided as well as sudden movements and change of position to reduce any chance of interference in the cardiac autonomic modulation.
After the guidance and positioning of the guardians and children, the collection occurred with the child at rest with spontaneous breathing for twenty-five minutes to collect HRV data. During collection, the following data was recorded: age, sex, weight, height, medications and placement on the waiting list for transplantation with the PELD score.
To collect HRV, heart rate was recorded beat by beat throughout the protocol with the cutoff frequency of 1,000 Hz by the Polar RS800Cx frequency meter (Polar Electro, Kempele, Finland) equipment previously validated for capturing the heart rate.
The same protocol was performed at two different times; pre- and post-surgical, in which the pre-surgical (Pre) was performed at the time the patient was already listed on the PELD with placement in the top ten. Post collection (Post) was performed at least 1 month (30 days) after LT. After that time, outpatient follow-up cards were evaluated for checking liver function tests.
The RR intervals were determined using digital filtering (Polar Precision Performance), and only one series with more than 95% sinus beats was included in the study. The Kubios HRV analysis software determined the indices used.
For data analysis, linear methods were divided into frequency and geometric time domains. The time domain were statistical analyses that take into account the intervals between r waves as a function of time. From the analysis, it is possible to observe the mean of the normal intervals (SDNN), the percentage of adjacent RR intervals with a difference greater than 50 ms in duration (PNN50), the square root of the mean of the sum of squares of the differences between adjacent RR intervals (RMSSD), among others (23).
SDNN can be obtained from both long-term and short-term recordings, and represents the modulation of all intrinsic parameters, for example circadian rhythm, which affect HRV as well as extrinsic ones such as physical activity (24). The RMSD, NN50 and pNN50 indices represent parasympathetic activity, obtained from the analysis of adjacent RR intervals (17).
The frequency domain or spectral analysis is the analysis of the power variance as a function of the frequency, and is represented as very low frequency (VLF), low frequency (LF), high frequency (HF), and total power (25).
The geometric indices presented here represent the global response of HRV where: RRTri (triangular index in which a histogram is shown, in which the horizontal axis is represented by all RR values, in a discrete scale; and in the vertical axis the frequency of occurrence of each) and triangular interpolation of NN interval histogram (TINN).
Statistical analysis
For quantitative variables, descriptive statistics were used to describe and characterize the data set, with distributions being presented in measures of central tendency and variability (mean and standard deviation).
The Shapiro-Wilk test was used to verify data distribution. For intragroup comparison, a t-test for paired measurements for normal distributions; and Wilcoxon test for non-normal distributions. The level of significance adopted was 5%. The statistical program used was Stata version 11.0.
Results
Initially, 25 guardians were approached (one of whom did not accept to participate in the study) of patients who participated in the pre-collection. One returned to the state of origin to carry out medical monitoring before performing the transplant, three died before performing the transplant, and three died shortly after transplantation (Figure 1). Altogether 17 patients completed the collections of the Pre and Post moments. The pre data was collected one week before surgery and the post analysis was at least one month after surgery and at the maximum two months after. Table 1 shows the characteristics of the patients.
Table 1
| Variables | Numbers |
|---|---|
| Age (months), | 25.29±29.84 |
| Gender, n (%) | |
| Female | 8 (47.06) |
| Male | 9 (52.94) |
| Diagnosis, n (%) | |
| Biliary pathway atresia | 15 (88.23) |
| Tyrosinemia | 1 (5.88) |
| Alagille’s syndrome | 1 (5.88) |
| Transplant, n (%) | |
| Living donor | 17 (100.0) |
| Deceased-donor | 0 (0) |
| PELD, | |
| PELD | 18±4 |
| Adjusted PELD | 54±12 |
| Prescripted drugs, | |
| Pre-transplant | 3±1 |
| Post-transplant | 6±3 |
PELD, pediatric end-stage liver disease.
The children were on average 2 years old at the time of pre-collection, 52.9% were male and 47% female. The initial diagnosis in 88.23% of the patients was biliary atresia (BA).
There was no difference between the diagnoses of BA, Alagille syndrome, portal thrombosis and tyrosinemia at the pre-moment regarding HRV indices, thus characterizing a homogeneous group.
Among the drugs prescribed, vitamins (52.9%), ursodeoxycholic acid (41.18%), and diuretic (41.18%) were among the most used, only one patient did not use any medication at the time of pre-collection. After transplantation, all (100%) used some type of immunosuppressant and 7 patients (41.18%) used hypotensive drugs, including beta-blockers, as shown in Table 1.
On average, patients used 3 medications before the transplant, while in the post-transplant period this average increased to 6 medications. The use of β-blockers showed no differences in the indices assessed when comparing patients who used the drug and those who did not, and there was a similarity between individuals in the pre- and post-transplant moments.
HRV analyzes were performed after digital filtering of the data, and the cutout used for the analysis was pre-defined at 1,000 points. With this number it is possible to perform different analyzes without prejudice to the result. The HRV averages at the pre moment are shown in Table 2.
Table 2
| Variables | Numbers () |
|---|---|
| Mean HR | 128.13±12.94 |
| Mean RR | 474.01±50.23 |
| SDNN | 19.66±11.65 |
| RMSSD | 9.45±5.03 |
| RRTri | 4.09±1.97 |
| TINN | 103.75±66.95 |
| PNN50 | 0.537±1.09 |
| VLFms2 | 189.78±198.36 |
| LFms2 | 94.26±96.10 |
| HFms2 | 19.51±21.52 |
| LF/HF | 4.94±3.48 |
| Total power | 266±198.01 |
HR, heart rate; RR, interval between two R waves; SDNN, standard deviation of the RR intervals; RMSSD, root-mean-square of the successive normal sinus RR interval difference; RRTri, triangular index; TINN, triangular interpolation of NN interval histogram; PNN50, percentage of adjacent RR intervals with a difference greater than 50 ms in duration; VLF, very low frequency; LF, low frequency; HF, high frequency.
When observing the results obtained by the linear analyses of the time domain, it was possible to notice an increase, but without significant difference in the mean heart rate, RMSSD, and PNN50 in the pre- to post-transplant moment. There was a significant increase in the SDNN index, which represents the global HRV modulation (Figure 2).
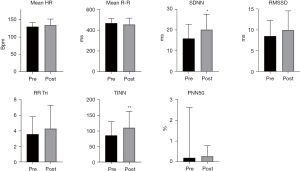
The TINN geometric index, calculated from the density histogram of the normal RR intervals, revealed a significant increase in the global variability from the Pre to the Post moment (Figure 2).
The Wilcoxon analysis performed on the frequency domain data showed a difference in the parameters of LFms2 (P=0.0372), HFms2 (P=0.0465), and the total frequency (P=0.0294) from the Pre to the Post moment. The increase in HFms2 represents the increase in the vagal response and the parasympathetic tone of the pre- to post-transplant moment. The increase in the LFms2 index and the total frequency indicate an increase in the overall HRV response (Figure 3). In the normalized units of the frequency domain indexes there were no significant differences.

Discussion
We found a parasympathetic predominance, which describes the improvement in modulation after surgical intervention. When analyzing the behavior of cardiac autonomic modulation in the period prior to LT and after surgery, an improvement in the global response of the ANS was noted by linear analyses of the time domain, frequency domain, and in the geometric domains. There was an increase in the global TINN index from the moment before transplantation to the moment after. Reflecting the vagal activity the HFms2 index showed an improvement (24).
There are several methods for diagnosing AD. In most cases, the tests involve invasive procedures, electrical stimuli, and the patients’ full collaboration; as in waltz tests, sweating, direct sympathetic nerve stimulation, and tilt test among others (26-28). Since these are non-collaborative children, the cardio-frequency meter proved to be the best instrument for assessing cardiac autonomic modulation.
Studies in children have demonstrated the influence of various diseases on cardiac autonomic modulation, such as obesity (28), diabetes mellitus, diabetic neuropathy (26,27), and Downs syndrome (29) in premature newborns (30) among others conditions, but there is no description of the influence of cirrhosis on HRV in children. Our results showed improvement in the parameters for SDNN, TINN, LFms2, HFms2, and total power of HRV after LT when compared with the same data before transplantation.
Chan and colleagues evaluated biochemical markers in conjunction with linear heart rate indices and HR complexity using multiple scales and entropy analysis. The evaluation of 30 adult individuals who underwent LT was performed before and after 1 year of transplantation. Chan concludes that compromised HRV in the pre-assessment is associated with higher post-transplant mortality and that the association of HRV with biochemical parameters is recommended for greater prognosis accuracy (31).
The analysis of HRV is an efficient non-invasive tool to assess the effects of liver disease on the cardiovascular system and in the diagnosis of AD (32-34). According to Keresztes et al., HRV collection for 24 hours proved to be more sensitive for the verification of sympathetic-vagal imbalance in patients with cirrhosis when compared to five standardized cardiovascular autonomic tests for this type of assessment (35).
AD is considered a risk of mortality in patients with acute or chronic liver disease (36). A study by Bhogal et al. evaluated HRV as a predictor of mortality in cirrhotic individuals, and reported a decrease in SD2 as an independent predictor of mortality in these patients; while SDNN is a predictor of Model for End-stage Liver Disease (MELD)-dependent mortality (37). The increase in this index found in the present study after LT suggests a decrease in the risk of mortality in transplant patients.
In a study by Ates et al. (38) in 2006, where the group compared the HRV of cirrhotic individuals with healthy individuals, it was concluded that the time domain indices of the linear HRV parameters are good aids in the prognosis of cirrhosis. Corroborating with the findings of the present study, the effect of LT on the SDNN index was evaluated by Baratta et al. (39). The evaluation was performed on 30 adults with cirrhosis followed by a 12-month follow-up, showing significant improvement of the SDNN one year after the transplant.
In the study by Baratta et al. (39) the evaluations carried out within 6 months of transplantation did not show significant improvement. In our evaluations, we could observe a significant increase in the SDNN index in a shorter period after surgery, which can be justified by the difference in the recovery process of cirrhotic children when compared to adults.
The post evaluation was performed a short time after LT (mean ± SD: 66±72 days), and significant differences were observed in the global HRV parameters such as SDNN, TINN, LFms2, and total power. In a study published by Tannuri et al., it was observed that live donor transplantation has a 25% longer survival in one year than patients who receive a cadaveric graft (40). All evaluated participants received the liver from living donors, which provided better control of the quality of the graft.
The improvement in HRV in the short term after the surgical intervention is predominantly attributed to the balance and recovery of hemodynamic, humoral, and metabolic systems after transplantation; and to a lesser extent the mechanical recovery of structural damage caused during surgery. The recovery of HRV parameters can be noticed even before the healing process is completed (10,39).
In this study, no differences were found in the indices that evaluate the short intervals, such as RMSSD and PNN50 (P=0.186 and P=0.687 respectively) that consider the adjacent intervals. Thus, we can understand that the difference in the global indices of this study was more influenced by the sympathetic system and less influenced by the parasympathetic one.
The sympathetic and vagal nervous systems can work in an agonist, antagonistic or independent manner, depending on the innervated organ and the function developed (41,42). Thus, the improvement in Total Power in the analysis of HRV frequency found in our study can be derived from the increase observed in both HFms2 and LFms2, indicating an improvement in the adaptation of the ANS after LT.
The assessment of HRV during several diseases has shown an imbalance of the sympathovagal system. In obese children, there is a reduction in parasympathetic; in diabetic children a reduction in the parasympathetic system has been detected as well as an overall reduction in HRV (27,28). In liver cirrhosis, parasympathetic dysfunction is more commonly detected than sympathetic dysfunction, this change is attributed to the greater involvement of parasympathetic fibers (43,44).
There was no difference in the linear time domain indices for parasympathetic modulations, the increase in vagal response was only observed by the frequency domain of the HFms2 index. This index is associated with respiratory cycles, so the respiratory frequency has a direct influence on this parameter (42,45).
The increase in HFms2 may be related to the improvement in the general condition of these children after LT, resulting from the improvement in respiratory mechanics after transplantation with a decrease in ascites, as well as the use of immunosuppressants, as presented by Sallan et al in their study experiment in which it reported an increase in HRV parameters correlated to the use of cyclosporin (46).
Non-specific beta-blockers are well established as a treatment for portal hypertension and ascites to prevent gastrointestinal bleeding. They act to reduce portal blood flow and reduce systemic vascular resistance without compromising blood pressure (47). Most studies consider the use of β blockers as exclusion factors due to the possible influence on HRV. The researchers responsible for the Task Force consider the changes found in the studies involving β blockers to be modest, although significant.
In this study, we did not interfere with the prescribed medications and chose not to exclude patients who used β blockers. When comparing the groups of patients who used β blockers with those who did not, we did not find significant differences, corroborating the study by Bhogal et al. who reported a difference in heart rate, but there was no difference in HRV when he compared 37 patients who took β blockers with 34 who did not take the medication (37).
In conclusion, the analysis of the behavior of cardiac autonomic modulation, in the period prior to LT and two months after surgery, showed an increase in linear HRV parameters SDNN, TINN, LFms2, and Total Power representing the overall improvement in HRV. In the time domain, there was also an increase in the HFms2 index, and parasympathetic tone of HRV.
Thus, the results presented here suggest an overall improvement in cardiac autonomic modulation. This global autonomic improvement presented indicates that, at the Pre moment, the children presented both a reduction in the parasympathetic system and an increase in the sympathetic system, and after the transplant, the balance of these systems was observed.
The time elapsed between transplantation and post-evaluation was shorter than those found in studies with transplanted cirrhotic adults (10,31,39), yet the results were similar with regard to the recovery of the ANS. This response positive in such a short time can be attributed to the ability to recover better and faster in children.
Thus, the study was performed in children with severe liver disease. The advanced stage of the disease with all the biochemical and physical alterations combined with the early ages made the data collection difficult. This study was the beginning of the investigation of cardiac autonomic responses in children with liver disease to identify parameters of HRV that could be predictors of mortality in pediatric patients. transplanted patients, studies that have already been carried out in adult patients.
Conclusions
There was an improvement in the autonomic modulation of heart rate and an increase in parasympathetic modulation in children with severe chronic liver disease after LT.
Acknowledgments
The authors thank nurses Helena Thie Miyatani and Paulo Renato Pereira (ICr – HC FMUSP) for assisting in the selection of patients at the liver transplant clinic. The authors also thank the attending physicians and residents of the liver transplant ambulatory at the Children’s Institute.
Funding: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) # 88881.131746/2016-01; Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) #2016/10570-7 #2017/2569-5.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-273/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-273/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-273/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethical committee of the Medicine School of the University of São Paulo (No.: 1.113.629) and informed consent was signed by the patients’ parents or legal guardians who were informed about the procedures and objectives of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chardot C. Biliary atresia. Orphanet J Rare Dis 2006;1:28. [Crossref] [PubMed]
- Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol 2014;20:7312-24. [Crossref] [PubMed]
- Hernaez R, Solà E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut 2017;66:541-53. [Crossref] [PubMed]
- Mattos ÂZ, Schacher FC, Mattos AA. Vasoconstrictors in hepatorenal syndrome - A critical review. Ann Hepatol 2019;18:287-90. [Crossref] [PubMed]
- Amin AA, Alabsawy EI, Jalan R, et al. Epidemiology, Pathophysiology, and Management of Hepatorenal Syndrome. Semin Nephrol 2019;39:17-30. [Crossref] [PubMed]
- Weinfurtner K, Forde K. Hepatopulmonary Syndrome and Portopulmonary Hypertension: Current Status and Implications for Liver Transplantation. Curr Hepatol Rep 2020;19:174-85. [Crossref] [PubMed]
- Soulaidopoulos S, Goulis I, Cholongitas E. Pulmonary manifestations of chronic liver disease: a comprehensive review. Ann Gastroenterol 2020;33:237-49. [Crossref] [PubMed]
- Dourakis SP, Geladari E, Geladari C, et al. Cirrhotic Cardiomyopathy: The Interplay Between Liver and Cardiac Muscle. How Does the Cardiovascular System React When the Liver is Diseased? Curr Cardiol Rev 2021;17:78-84. [Crossref] [PubMed]
- Rugină M, Predescu L, Sălăgean M, et al. Pre-liver transplantation, cardiac assessment. Chirurgia (Bucur) 2012;107:283-90. [PubMed]
- Di Stefano C, Milazzo V, Milan A, et al. The role of autonomic dysfunction in cirrhotic patients before and after liver transplantation. Review of the literature. Liver Int 2016;36:1081-9. [Crossref] [PubMed]
- Trevisani F, Caraceni P, Simoncini M, et al. Evidence of oxidative imbalance in long-term liver transplant patients. Dig Liver Dis 2002;34:279-84. [Crossref] [PubMed]
- Bajaj BK, Agarwal MP, Ram BK. Autonomic neuropathy in patients with hepatic cirrhosis. Postgrad Med J 2003;79:408-11. [Crossref] [PubMed]
- Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet 1992;339:1462-4. [Crossref] [PubMed]
- Pérez-Peña J, Rincón D, Bañares R, et al. Autonomic neuropathy is associated with hemodynamic instability during human liver transplantation. Transplant Proc 2003;35:1866-8. [Crossref] [PubMed]
- Tannus LR, Drummond KR, Clemente EL, et al. Predictors of cardiovascular autonomic neuropathy in patients with type 1 diabetes. Front Endocrinol (Lausanne) 2014;5:191. [Crossref] [PubMed]
- Rajendra Acharya U, Paul Joseph K, Kannathal N, et al. Heart rate variability: a review. Med Biol Eng Comput 2006;44:1031-51. [Crossref] [PubMed]
- Vanderlei LCM, Pastre CM, Hoshi RA, et al. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Rev Bras Cir Cardiovasc 2009;24:205-17. [Crossref] [PubMed]
- Goldenberg I, Goldkorn R, Shlomo N, et al. Heart Rate Variability for Risk Assessment of Myocardial Ischemia in Patients Without Known Coronary Artery Disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study. J Am Heart Assoc 2019;8:e014540. [Crossref] [PubMed]
- Amaral JATD, Salatini R, Arab C, et al. Non-Alcoholic Cirrhosis and Heart Rate Variability: A Systematic Mini-Review. Medicina (Kaunas) 2020;56:116. [Crossref] [PubMed]
- Carey EJ, Gautam M, Ingall T, et al. The effect of liver transplantation on autonomic dysfunction in patients with end-stage liver disease. Liver Transpl 2008;14:235-9. [Crossref] [PubMed]
- Pérez-Peña J, Rincón D, Bañares R, et al. Autonomic neuropathy in end-stage cirrhotic patients and evolution after liver transplantation. Transplant Proc 2003;35:1834-5. [Crossref] [PubMed]
- da Saúde M. Regulamento Técnico do Sistema Nacional de Transplantes. PORTARIA No 2.600. [cited 2017 Mar 5].
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354-81. [Crossref] [PubMed]
- Xhyheri B, Manfrini O, Mazzolini M, et al. Heart rate variability today. Prog Cardiovasc Dis 2012;55:321-31. [Crossref] [PubMed]
- Kleiger RE, Stein PK, Bigger JT Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005;10:88-101. [Crossref] [PubMed]
- Chessa M, Butera G, Lanza GA, et al. Role of heart rate variability in the early diagnosis of diabetic autonomic neuropathy in children. Herz 2002;27:785-90. [Crossref] [PubMed]
- Giacon TR, Vanderlei FM, Christofaro DG, et al. Impact of Diabetes Type 1 in Children on Autonomic Modulation at Rest and in Response to the Active Orthostatic Test. PLoS One 2016;11:e0164375. [Crossref] [PubMed]
- Vanderlei LCM, Pastre CM, Freitas Júnior IF, et al. Índices geométricos de variabilidade da frequência cardíaca em crianças obesas e eutróficas. Arq Bras Cardiol 2010;95:35-40. [Crossref] [PubMed]
- Carvalho TD, Massetti T, Silva TDD, et al. Heart rate variability in individuals with Down syndrome - A systematic review and meta-analysis. Auton Neurosci 2018;213:23-33. [Crossref] [PubMed]
- Selig FA, Tonolli ER, Silva EV, et al. Heart rate variability in preterm and term neonates. Arq Bras Cardiol 2011;96:443-9. [Crossref] [PubMed]
- Chan KC, Yeh JR, Sun WZ. The role of autonomic dysfunction in predicting 1-year mortality after liver transplantation. Liver Int 2017;37:1239-48. [Crossref] [PubMed]
- Fleisher LA, Fleckenstein JF, Frank SM, et al. Heart rate variability as a predictor of autonomic dysfunction in patients awaiting liver transplantation. Dig Dis Sci 2000;45:340-4. [Crossref] [PubMed]
- Negru RD, Cojocaru DC, Mitu F, et al. Contribution of the heart rate variability parameters in evaluation of liver cirrhosis severity and associated autonomic dysfunction. Acta Medica Mediterr 2015;31:1087-92.
- Osztovits J, Horváth T, Abonyi M, et al. Chronic hepatitis C virus infection associated with autonomic dysfunction. Liver Int 2009;29:1473-8. [Crossref] [PubMed]
- Keresztes K, Istenes I, Folhoffer A, et al. Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World J Gastroenterol 2004;10:3039-43. [Crossref] [PubMed]
- Milovanovic B, Milinic N, Trifunovic D, et al. Autonomic dysfunction in alcoholic cirrhosis and its relation to sudden cardiac death risk predictors. Gen Physiol Biophys 2009;28:251-61. [PubMed]
- Bhogal AS, De Rui M, Pavanello D, et al. Which heart rate variability index is an independent predictor of mortality in cirrhosis? Dig Liver Dis 2019;51:695-702. [Crossref] [PubMed]
- Ates F, Topal E, Kosar F, et al. The relationship of heart rate variability with severity and prognosis of cirrhosis. Dig Dis Sci 2006;51:1614-8. [Crossref] [PubMed]
- Baratta L, Tubani L, Merli M, et al. Long-term effect of liver transplantation on cirrhotic autonomic cardiac dysfunction. Dig Liver Dis 2010;42:131-6. [Crossref] [PubMed]
- Tannuri AC, Porta G, Kazue Miura I, et al. Pediatric acute liver failure in Brazil: Is living donor liver transplantation the best choice for treatment? Liver Transpl 2016;22:1006-13. [Crossref] [PubMed]
- Wehrwein EA, Orer HS, Barman SM. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol 2016;6:1239-78. [Crossref] [PubMed]
- Karemaker JM. An introduction into autonomic nervous function. Physiol Meas 2017;38:R89-R118. [Crossref] [PubMed]
- Trevisani F, Sica G, Mainquà P, et al. Autonomic dysfunction and hyperdynamic circulation in cirrhosis with ascites. Hepatology 1999;30:1387-92. [Crossref] [PubMed]
- Ewing DJ, Campbell IW, Clarke BF. Heart rate changes in diabetes mellitus. Lancet 1981;1:183-6. [Crossref] [PubMed]
- Heathers JA. Everything Hertz: methodological issues in short-term frequency-domain HRV. Front Physiol 2014;5:177. [Crossref] [PubMed]
- Sallam MY, El-Gowilly SM, Abdel-Galil AA, et al. Cyclosporine counteracts endotoxemia-evoked reductions in blood pressure and cardiac autonomic dysfunction via central sGC/MAPKs signaling in rats. Eur J Pharmacol 2017;797:143-52. [Crossref] [PubMed]
- Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol 2017;66:849-59. [Crossref] [PubMed]



