
Pharmaceutical services based on therapeutic care pathway for kidney transplantation from donors of infants and young children: a single-center experience
Introduction
Kidney transplantation is the preferred treatment for children with end-stage renal disease (ESRD), which has substantial benefits including the best chances for growth and better quality of life (1). Unfortunately, access to kidney transplantation for children with ESRD is limited due to the scarcity of donor’s kidneys. An analysis from the International Pediatric Nephrology Association Global Kidney Replacement Therapy Registry indicates that using kidneys donated by infants and young children can result in successful transplant outcomes (2). It is reported that single kidney transplantation from pediatric donors weighing 5–10 kg to pediatric recipients is feasible and has favorable outcomes (3). Thus, the application of kidneys donated by infants and young children may be a possible way to expand the transplantation pool.
Due to the specificity of pediatric donors and recipients, the difficulty of the procedure, and the complexity of postoperative complications, kidney transplantation from donors of infants and children to pediatric recipients is particularly challenging. It needs a multidisciplinary collaboration (4), including monitored anesthesia care, clinical care, pharmaceutical services, etc. To regulate the pharmaceutical services, it is necessary to establish procedures. Therapeutic care pathway, which is known as a part of Diagnostic-Therapeutic Care Pathways (DTCPs) (5), is a predefined sequence of therapeutic and assistance activities that integrates the participation of several specialists to obtain the most appropriate therapy for each patient. Pharmaceutical services based on therapeutic care pathway may be a novel method to improve the survival rate of patients. The past decade has witnessed the rapid development of pharmaceutical services and DTCPs in many areas. However, limited data exist to demonstrate the effect of incorporating patient-centered care using concepts of therapeutic care pathways by clinical pharmacists for pediatric recipients receiving their kidneys from infants and young children.
The development of immunosuppressive regimens has improved treatment outcomes. The post-transplant immunosuppression in the modern era includes two phases: induction immunosuppression which is administered in the perioperative period to prevent early rejection, and maintenance immunosuppression that is administered indefinitely to promote long-term graft survival (6). Induction agents include lymphocyte-depleting antibodies such as rabbit antithymocyte globulin, alemtuzumab, muromonab-CD3, rituximab, and bortezomib; lymphocyte-nondepleting antibodies, and other discontinued or investigational agents. Maintenance immunosuppression includes two or more immunosuppressive agents used in combination to achieve synergy and to limit treatment-related toxicity (6). For example, one of the immunosuppressive agents, calcineurin inhibitors—including cyclosporine and tacrolimus (TAC), could significantly improve the short-term renal graft survival by lowering the acute rejection rates (7). The immunosuppressive regimens with the fewest possible toxic effects are desirable for both the pediatric recipients and the doctors. Therefore, providing careful pharmaceutical services for those recipients with different physiological and biochemical parameters from the adults is considerably important, given the need to maintain optimal graft function and survival while managing the debilitating side effects and medication associated complications.
In this report, we retrospectively reviewed the clinical outcomes of kidney transplantation from infants and young children to pediatric recipients between 2011 and 2013. We shared our patient-centered pharmaceutical services based on the three-step protocol for kidney transplantation from donors of infants and young children, by collecting and reviewing their clinical data, including the clinical characteristics, outcome indices, and follow-up data from all patients. It ensured that clinical pharmacists possess the core competencies which is necessary to contribute meaningfully to the optimal use of medications and patient outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-515/rc).
Methods
Study population
This was a retrospective, observational, single-center study in which we described seven single and five bilateral-kidney transplantation from small pediatric donors in our Organ Transplant Center between September 2011 and December 2013. All pediatric patients who underwent kidney transplantation from donors of infants and young children were enrolled in a retrospective database. The study was ethically reviewed and approved by the Ethics Committee of the Changzheng Hospital, Naval Medical University (No. 2019SL021), and informed consent was obtained from each patient or their family members. All donations were voluntary, unpaid, and no organs were obtained from prisoners. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Therapeutic care pathway
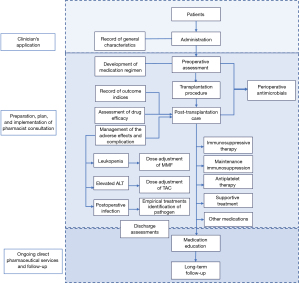
The overall flow chart of the study is shown in Figure 1. The clinical characteristics of recipients were retrieved from the kidney transplantation database and electronic medical record system in our hospital. All surgeries were performed according to the latest guidelines by experienced surgeons. Post-operative management included the performance of medication regimen, therapeutic drug monitoring (TDM), assessment of drug efficacy, and management of the adverse effects. The patients can be discharged from the hospital when the graft function recovered and all catheters were removed. Besides, the TAC blood concentration, the levels of the serum index for liver function and infection should be in the normal range.
The induction therapy post-transplantation included anti-CD25 monoclonal antibodies plus methylprednisolone, and the maintenance immunosuppression composed a steroid-free regimen with TAC and mycophenolate mofetil (MMF). The patients were intravenously administered with 1 mg/kg/d of anti-CD25 monoclonal antibodies intra-operatively and then administered at 7 days and 14 days after surgery. Methylprednisolone dose was gradually decreased once daily with the starting dose of 10 mg/kg/d, until the drug was discontinued during the initial 7 days. During the initial post-operative phases (about 3 days), TAC was given by intravenous injection to obtain a stable blood concentration. The oral dose of TAC was determined according to the CYP3A5 genotype of the recipients, and MMF was administered initially as 500–1,000 mg/d based on the patient’s weight. Prophylactic anticoagulation with heparin (5–10 U/kg/h) was initiated immediately after the surgery, with a dosage adjusted according to the coagulation function (APTT) test (target value 28–40 s). Subcutaneous heparin was replaced by oral aspirin (100 mg, per night) at 1-week post-operation. As urinary tract infection remained the commonest source of systemic infection among kidney transplant recipients (8), piperacillin (100 mg/kg) plus tazobactam (12.5 mg/kg) was prescribed pre-transplantation to prevent infection as empirical anti-microbial policy, and was used until the 7th day of post-operative period.
Outcome measures
The primary outcome was TAC trough blood concentration, and the secondary outcomes included graft function (SCr), liver function (ALT and AST), infection indicators (CD4 and WBC), the occurrence of undesirable effects and postoperative complications, and long-term clinical indices such as the growth and development of the patients during the follow-up. When adequate urine flow was established immediately with a progressive decline in serum creatinine reaching normal levels (<130 µmol/L), early graft function was considered excellent. Delayed graft function (DGF), presenting as suboptimal renal function immediately following kidney transplantation, was a manifestation of ischemia-reperfusion injury in the transplanted kidney allograft. TDM of TAC was applied regularly to all recipients and dose adjustment was performed to achieve target trough levels (6–10 ng/mL). The required days of reaching steady-state blood concentration referred to the days that TAC blood concentration needed to reach the above-mentioned target range of concentration. Genotyping of CYP3A5 (A6986G) was determined by polymerase chain reaction. MMF dose was also optimized by TDM, aiming to adjust the trough concentrations of mycophenolic acid (the active metabolite of MMF) within 1–1.5 µg/mL (9), which was monitored by enzyme multiplied immunoassay technology method.
Role of clinical pharmacists
Clinical pharmacists’ interventions can improve the clinical outcomes. In 2016, the American Society of Health-System Pharmacists’ Pharmacy Practice Model Initiative recommended to develop and disseminate pharmacy practice models that optimize the use of pharmacists as direct care providers (10). In this study, clinical pharmacists were the policymakers of medication protocols, the implementers of pharmaceutical services, and the consultant of medication education. Pharmacists working in the Organ Transplant Center are skilled in perioperative medicine management of transplant patients and have more than 5 years of work experience. In China, pharmaceutical services developed rapidly recent years since the Chinese Ministry of Health firstly proposed to establish a clinical pharmacist system in hospitals in 2002 (11). A recent systematic review has shown that in China, the pharmacist-led intervention could significantly reduce the length of hospitalization, readmission rate and mortality during hospital of patients (12). Due to the personal shortage in majority of hospitals, it is unrealistic to apply personal-centered pharmaceutical services to all patients (13). In our hospital, clinical pharmacists provide pharmaceutical services for the patients who are treated with cardiology, nephrology, endocrinology, respiratory medicines, as well as those who received organ transplantation. Moreover, a medication consultation clinic is set up to provide inpatient and outpatient consultations to all requesting pharmaceutical services.
Combined with evidence-based medicine and clinical pathway methods, we established a three-step-protocol of pharmaceutical services to regulate the formulation procedure and specify the main content of the pharmaceutical care procedure. The three-step protocol for the kidney transplantation patients was as follows:
- Clinician’s application—the clinicians applied for a pharmacist consultation in the electronic system, then confirmed by the pharmacist.
- Preparation, plan, and implementation of pharmacist consultation—before each pharmacist consultation, the clinical pharmacists had good preparation for understanding the patient's clinical status, observed and evaluated the patient's medication-related needs, assessed the potential drug-related problems, and then developed an initial treatment plan.
- For each patient, after face-to-face communicating with the patient and collecting clinical information, the pharmacist conducted a custom-designed medication guideline (time, methods, attentions), and provided related pharmacotherapy knowledge.
- The pharmacist optimized the patient’s medication regimen based on the patient’s baseline characteristics (such as weight, renal function, and CYP3A5 gene type), which could prevent possible medication-related problems (such as thrombosis, postoperative infection, and leukopenia).
- The pharmacist could identify and resolve the medication-related problems, and communicate effectively with the patients. A cross-sectional prospective study had shown that a consistent post-transplantation pharmaceutical care service is effective to substantially improve knowledge of post-transplantation self-care. Pharmaceutical care should be started as early as possible during the pre-transplant period and continue in a long-term follow-up (14). For each patient, the pharmacist evaluated the potential risks in clinical practice, and answered the drug-related consultation questions. Indicators that were relevant to the undesirable effects and postoperative complication, especially the concentration of drugs were paid close attention. By timely adjustment of the contents of the pharmaceutical services with the change of the patient’s condition and drug treatment plan, the pharmacist could make sure that the usage and dosage of drugs were appropriate.
- Ongoing direct pharmaceutical services and follow-up—as complex pharmacotherapy regimens for immunosuppression remain necessary for graft survival in the solid organ transplant recipient, and many patients remain on lifelong dual immunosuppression (15), ongoing follow-up is necessary. For all patients, the clinical pharmacists enquired their clinical characteristics, medications, outcome indices, etc. through the ongoing direct pharmaceutical care and follow-up. Furthermore, the specific pharmacist interventions also included consultation-based health education and drug optimization.
Statistical analysis
Statistical analysis was performed using the SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation and were compared using either the unpaired Student’s t-test or ANOVA tests. Categorical variables were expressed as numbers and percentages and were compared using the chi-square test or Fisher’s exact test, as appropriate. P values <0.05 were considered statistically significant.
Results
Characteristics of recipients
There were five male and seven female recipients. The major cause of ESRD (33.3%) was chronic glomerulonephritis (Table 1). Their mean age at the time of transplantation was 8.73±2.91 years (range, 4.6–14.8). The mean weight of recipients was (21.79±6.52) kilograms (range, 14–37.5). The mean height of recipients was (115.83±14.90) centimeters (range, 95–143). The average hospitalization days were 43±19.34 days (median, 42.5). All patients received peritoneal dialysis before transplantation.
Table 1
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height, cm | 105 | 129 | 120 | 95 | 143 | 110 | 97 | 125 | 120 | 133 | 105 | 108 |
| Weight, kg | 18 | 29 | 23.5 | 21.5 | 37.5 | 20 | 15 | 23.5 | 20.5 | 24 | 14 | 15 |
| Primary kidney disease | NS | CGN | CGN | Congenital renal insufficiency | CGN | Mesangial proliferative glomerulonephritis | Kearns-Sayre syndrome | Bilateral ureteral regurgitation | Glomerular mesangial hyperplasia | CGN | Unknown | NS |
| Hospital stay, d | 2012/2/2 | 2012/12/25 | 2013/3/19 | 2013/3/3 | 2013/3/3 | 2013/5/29 | 2013/5/29 | 2013/11/16 | 2013/12/20 | 2013/12/20 | 2011/9/13 | 2013/2/4 |
| 2012/4/12 | 2013/2/19 | 2013/5/20 | 2013/5/7 | 2013/5/6 | 2013/6/17 | 2013/6/17 | 2014/1/4 | 2014/1/20 | 2014/1/18 | 2011/9/29 | 2013/3/12 | |
| 70 | 56 | 62 | 65 | 64 | 19 | 19 | 49 | 31 | 29 | 16 | 36 | |
| Hospital costs, CNY (Y) | 153,338.37 | 103,541.91 | 103,711.3 | 68,402.69 | 78,455.92 | 47,933.63 | 48,786.85 | 58,566.63 | 65,268.05 | 54,451.22 | 37,886.07 | 85,490.09 |
| 96,063.37 | 51,577.71 | 54,511.38 | 29,031.29 | 35,896.12 | 22,615.13 | 22,622.75 | 25,175.03 | 32,938.45 | 25,904.22 | 13,430.07 | 30,670.09 | |
| PRA | – | – | – | – | – | – | – | – | – | – | – | – |
| Dialysis modality | PD | |||||||||||
| Operation name | Allogeneic kidney transplantation | |||||||||||
| Incision type | Class II | |||||||||||
| Donor kidney situation | Bilateral | Bilateral | Bilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral | Bilateral | Bilateral |
NS, nephrotic syndrome; CGN, chronic glomerulonephritis; PD, peritoneal dialysis; PRA, plasma renin activity; d, day.
Outcome of recipients
Primary outcome
The Immunosuppressive regimen is shown in Table 2. No statistical significance was observed in TAC concentration/dose between the two CYP3A5 genotypes (AG and GG) by t-test (P>0.05, P1w=0.992, P2w=0.323, P4w=0.167).
Table 2
| Immunosuppressive regimen post-transplantation | Case 1 | Case 2 | Case 7 | Case 3 | Case 5 | Case 6 | Case 9 | Case 4 | Case 8 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight, kg | 18 | 29 | 15 | 23.5 | 37.5 | 20 | 20.5 | 21.5 | 23.5 | 24 | |
| CYP3A5 | − | − | − | GG | GG | GG | GG | AG | AG | AG | |
| Immune induction scheme | Anti-CD25 monoclonal antibody, mg | 25 | 50 | 25 | 25 | 50 | 25 | 25 | 25 | 25 | 25 |
| MP, mg | 160 | 240 | 120 | 200 | 240 | 160 | 160 | 160 | 160 | 160 | |
| Immune maintenance scheme | TAC, mg | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| MMF, mg | 360 | 540 | 360 | 540 | 540 | 360 | 540 | 360 | 540 | 540 | |
| Blood TAC concentration/dose | 1 week after administration | – | 0.42±0.29 | 0.50±0.25 | |||||||
| 2 weeks after administration | 0.20±0.089 | 0.15±0.025 | |||||||||
| 4 weeks after administration | 0.23 ± 0.11 | 0.26±0.024 | |||||||||
MP, methylprednisolone; TAC, tacrolimus; MMF, mycophenolate mofetil; mg, milligram.
The results from stepwise multiple regression suggested that the time required for steady-state concentration of TAC was correlated with the CYP3A5 genotype (P=0.0464) (Table 3).
Table 3
| Variable | B | SE | Partial R2 | R2 | F | P |
|---|---|---|---|---|---|---|
| Gene | −3.3333 | 1.41593 | 0.4092 | 0.4092 | 5.54 | 0.0464 |
Secondary outcome
Periprocedural complications occurred in 10 of 12 patients (83.3%). The most frequent complication was bleeding (25%) and DGF (25%) (Table 4). Acute rejection occurred in one recipient 19-day post-transplantation. The renal function resumed to be normal by pulse therapy with methylprednisolone and maintenance therapy with peritoneal dialysis. Other complications included urine leaks (cases 1 and 3), hydronephrosis (case 4), nephredema (case 5), and pulmonary infection (cases 2 and 7). Graft loss was found in two patients. The first graft loss occurred 2 days post-transplantation in case 11 due to thrombosis. The second graft loss occurred 10 days post-transplantation in case 12 due to peritonitis.
Table 4
| Outcomes of recipients | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complications after transplantation | ||||||||||||
| Acute graft rejection | d19 | |||||||||||
| DGF | d3 | d3 | d3 | |||||||||
| Thrombosis | d2 | |||||||||||
| Bleeding | d12 | d4 | d2 | |||||||||
| Urinary fistula | d23 | d14 | ||||||||||
| Hydronephrosis | d21 | |||||||||||
| Nephredema | d20 | |||||||||||
| Pulmonary infection | d5 | d2 | ||||||||||
| Peritonitis | d10 | |||||||||||
| Adverse drug reaction | ||||||||||||
| Elevated ALT and/or AST | d25 | d7 | ||||||||||
| Gross hematuria | d22 | |||||||||||
| Leukopenia | d13 | d47 | d15 | d12 | d3 | |||||||
| Reduced urine output and increased creatinine | d9 | |||||||||||
| Increased uric acid | d15 | |||||||||||
| Long-term prognosis (one year after operation) | ||||||||||||
| Increase in height, cm | 10 | 11 | 15 | 20 | 6 | 14 | 13 | 1 | 1 | 1 | − | − |
| Increase in weight, kg | 7 | 9 | 5.4 | 1 | 8 | 5 | 3 | 1.5 | 1.5 | 1 | ||
| Proteinuria | ± | + | - | - | + | - | - | - | - | - | ||
| 24-hour urine output, mL | 1,000 | 1,300 | 1,200 | 700 | 1,400 | 1,000 | 1,000 | 1,200 | 1,450 | 1,000 |
DGF, delayed graft function; d19 means the day 19 after kidney transplantation; d, day, ALT, alanine aminotransferase; AST, aspartate aminotransferase; cm, centimeter; kg, kilogram; ml, milliliter.
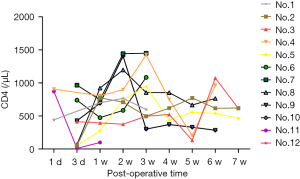
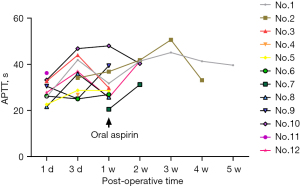
Figure 2 shows the change of blood concentration of TAC, levels of AST, ALT, and SCr of the post-transplantation patients. The average SCr at 1-week post-operation was 344.5±222.1 µmol/L, which could be regarded as the sign of recovered renal function when decreased to 130 µmol/L. The results from logistic regression analyses suggested that no statistical significance was observed between the recovery of renal function and either the dose of anti-CD25 monoclonal antibodies, methylprednisolone, TAC, or MMF. The CD4, WBC as well as APTT levels after the transplantation are shown in Figures 3-5.
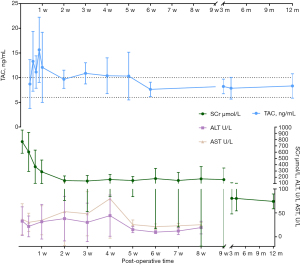
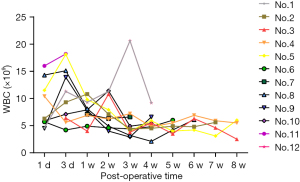
Increased levels of ALT and AST were observed in two patients, whose blood concentrations of TAC were both above 20 ng/mg. After dose adjustment, both levels decreased (Figure 2). Leukopenia was observed in five of the ten successfully transplanted patients, which was considered to be partly associated with MMF (Figure 4). Therefore, those patients received MMF dose adjustment and symptomatic supportive therapy. Of note, the immunosuppressant of one patient was changed from MMF to mizoribine. However, this patient stopped using MMF again owing to the elevated uric acid by mizoribine. In addition to this, other side effects such as gross hematuria, reduced urine output, and increased creatinine were also observed in other patients.
10 successfully transplanted patients were followed up for 1-year post-operation to ensure good adherence. The average height of the patients changed from 117.9±16.11 to 127.1±14.10 cm (the increase ranged 1–15 cm), and the average weight changed from 23.25±6.27 to 27.52±8.19 kg (the increase ranged 1–9 kg). Only 2 patients showed qualitative proteinuria, with an average 24 h urine volume of 1,125±324 mL. However, the symptom of proteinuria improved after symptomatic treatment with Huangkui capsule.
Discussion
Major finding
Kidney transplantation is the ideal form of renal replacement therapy. Since it was first performed in pediatric patients in the 1960s and 1970s (16,17), the success rate of kidney transplantation has been constantly increasing with the development of surgical techniques and new immunosuppressive agents, as well as the postoperative care of pediatric kidney transplant patients (18,19). Based on the guidelines for transplant perioperative treatment, we summarized a perioperative patient-centered clinical pharmaceutical services based on the three-step protocol for 12 pediatric kidney transplantation patients, whose mean age at the time of transplantation was 8.73±2.91 years, established an internet follow-up system, and achieved a good outcome.
Law of pharmacy
Table 5 provides a summary of recommendations in patients. It is important to emphasize here that the drugs listed in the table were the most, but not all drugs that used in the therapy. Other treatments such as liver protection treatment, anti- anemia treatment, and symptomatic supportive treatment were equally important.
Table 5
| Therapy | Drug name | Dose | Administration time (post-operation, d) | Drug administration | Target concentration |
|---|---|---|---|---|---|
| Inductive immunosuppressive therapy | Anti-CD25 monoclonal antibody | 1 mg/kg/d | 0, 7, 14 | Intravenous | |
| Methylprednisolone | 10 mg/kg/d | 0–7 | Intravenous | ||
| Maintenance immunosuppression therapy | TAC | 0.1 mg/kg/d | 0–1 | 24-h continuous infusion | 6–10 ng/mL |
| 1– | Oral | ||||
| MMF | 360–540 mg | 0– | Oral | 1–1.5 µg/mL | |
| Antiplatelet therapy | Heparin | 5–10 U/kg/h | 0–7 | 24-h continuous infusion | |
| Aspirin | 100 mg | 7– | Oral (per evening) | ||
| Antimicrobial therapy | Piperacillin | 100 mg/kg/d | −1–7 | Intravenous | |
| Tazobactam | 12.5 mg/kg/d | −1–7 | Intravenous |
TAC, tacrolimus; MMF, mycophenolate mofetil; mg, milligram; kg, kilogram; d, day; U, unit; h, hour; ng, nanogram; µg, microgram.
Inductive immunosuppressive therapy
Inductive immunosuppressive therapy consists of initial induction and maintenance regimens to prevent rejection. Induction may be defined as treatment with a biologic agent either before, at the time of, or immediately after transplantation to deplete or modulate T cell responses at the time of antigen presentation (20). Bunnapradist et al. (21) retrospectively analyzed the United Network for Organ Sharing Data to identify factors associated with the use of induction therapy and classes of induction agents used for kidney transplant recipients. The authors concluded that antibody induction was associated with a lower risk of rejection and better graft survival. A recent randomized controlled trial (22) concluded that among patients at high risk for acute rejection, induction therapy consisting of a 5-day course of antithymocyte globulin, as compared with basiliximab, reduced the incidence and severity of acute rejection. Considering that acute rejection is more frequent in children than in adults (23), we chose anti-CD25 monoclonal antibodies and methylprednisolone as the induction drugs and adopted a normal dose of anti-CD25 monoclonal antibodies of 1 mg/kg/d. The dose of methylprednisolone was calculated according to the patient’s weight at an original dose of 10 mg/kg/d, then gradually decreased by 40 mg/d until withdrawal. In our study, the incidence of acute rejection was 1/12 (Table 4). This patient was assessed again by the clinic pharmacist and then treated with peritoneal dialysis and methylprednisolone pulse therapy. The incidence could be reduced by optimizing the immune induction regimen in the future.
Maintenance immunosuppression therapy
Maintenance immunosuppression uses multiple medications to target different regions of the immune response to prevent rejection or graft versus host disease (GVHD) (24). In a randomized trial (25), Trompeter et al. demonstrated that TAC was significantly more effective than cyclosporine in preventing acute rejection after kidney transplantation in a pediatric population. As stated in the annual report issued by the NAPRTCS (26), a TAC-MMF-prednisone or TAC-MMF combination was the most common maintenance immunosuppression regimen used. Several studies had documented that complete steroid avoidance was safe in children receiving primary kidney transplants (27-29). Considering the adverse effects of steroids, we chose a TAC-MMF combination regimen in this study. TAC is a widely used immunosuppressive medication with a narrow therapeutic index and large between-patient pharmacokinetic variability, with a half-life of approximately 12 to 18 hours. Many researches have evidenced the link of CYP3A5 polymorphism with the inter-individual variability in oral TAC disposition. As Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines recommended, extensive metabolizers (CYP3A5*1/*1) in the genotyped-dosed group had an increase in TAC dose to 0.3 mg/kg/day, whereas the poor metabolizers (CYP3A5*3/*3) had a decrease to 0.15 mg/kg/day, and the intermediate metabolizer (CYP3A5*1/*3) received 0.2 mg/kg/day (30). However, all patients were administered TAC as a continuous i.v. infusion at 0.1 mg/kg/d after the surgery for about at least 3 days to ensure its steady-state blood concentrations when genotype information is not available (31). Then, patients were transferred to oral TAC, receiving the initial oral dose of which guided by the CYP3A5 polymorphism. The subsequent dose individualization for both TAC and MMF were adjusted according to clinical pharmacist judgement, depending on the serum level. Of note, TAC therapy could be associated with nephrotoxicity, resulting in long-term renal dysfunction, hypertension, and hyperlipidemia. Thus, the serum index for liver and renal functions should be in regular monitoring. Figure 2 showed both the information of the SCr value and TAC blood level, the same change tendency of the two indicators could help for the understanding of the relationship between them. Additionally, the variance of TAC trough blood level gradually becomes smaller over time, suggesting the efficient TAC therapy management by the pharmaceutical services.
Antiplatelet therapy
Graft thrombosis is the most common cause of early graft failure in pediatric kidney transplantation (32). The rate of graft loss due to thrombosis is significantly higher in younger children (less than 2 years of age) as compared with older age groups (33). In a clinical trial (34), Nasrin Esfandiar revealed a reduction in kidney allograft thrombosis incidence in children who received heparin and aspirin after transplantation. The American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (35) suggested that all children, in light of the individual risk factors for bleeding, and the perceived risk of the thrombosis, will require individualization of the initial dosing strategy of unfractionated (or standard) heparin. When aspirin is used for antiplatelet therapy in children, it is used in doses of 1 to 5 mg/kg per day (Grade 2C). In our study, heparin was administrated intravenously at an original dose of 5–10 U/kg/h, then it was switched to oral aspirin (100 mg, each night). As seen in Figure 5 and Table 4, although APTT in the patients was at normal-range levels, there were 3 cases of bleeding and 1 case of thrombosis. A possible explanation was that extrapolating the APTT range from adults to pediatric patients was unlikely to be valid (35). Strategies to enhance postoperative pharmaceutical monitoring might involve employing thrombelastography and enhancing monitoring.
Antimicrobial therapy
Regarding the use of antimicrobials, we chose piperacillin (100 mg/kg) combined with tazobactam (12.5 mg/kg) as the regular postoperative medication. Reasons for this were that allograft kidney transplantation was a type II incisional procedure, so the procedure time was always long, and the graft could be regarded as an implanted foreign body. Furthermore, considering the ICU stay of donors, we could not exclude the possibility of contamination with multidrug-tolerant bacteria, so empiric broad-spectrum antimicrobial coverage was advocated. Besides, the most often postoperative infections, such as urinary tract infection and pulmonary infection, managed by the initial administration of empirical antibiotics covering both Gram-negative and Gram-positive bacteria, and specific therapy is thereafter initiated when identifying the pathogen by the available culture results. Figure 3 showed a visual description for the change of CD4 values, which is an indicator of infection, especially for the lung infection. Similarly, Figure 4 depicted the trend of WBC levels, which is useful for the inflammation evaluation. When the patients showed increased WBC levels (>10×109/L), empirical antibiotics combines with specific therapy could be considered. For example, urinary tract infection presenting with clinical features of acute pyelonephritis should be treated for at least 10–14 days, until the pathogen had been eradicated by assessment of infection samples after a certain follow-up (36,37). Furthermore, as post-transplant immunosuppression unnecessarily posed the patient to an increased risk of infection in general, close attention should be paid to the immunosuppressant dosage and treatment regimen, the individualization of the drug therapy would help to allow more effective antimicrobial potency of the host immune system.
Long-term follow-up
As regards the follow-up of the successful patients, we designed a series of questionnaires (Appendix 1,2) to gain more insight into the growth and development, postoperative recovery, and medication adherence of the patients. Our pharmacy internet follow-up system was established on the basis of the follow-up system for the donors and the recipients. Three main functions were designed to achieve our goal of long-term follow-up: data entry (export), remote communication, and a personalized short message service reminder. The establishment of this system could improve patient adherence, and promote the accumulation of clinical data. Follow-up on the patients postoperatively showed a significant improvement in the growth and development, and the transplants were successful.
Clinical consideration
As a member of the clinical team, clinical pharmacists play a significant role throughout the process of transplantation. Before the operation, we participate in the pre-operative assessments of the patients, and establish the individualized medication protocol. After the operation, we adjust treatment plans in response to changes during the postoperative therapy, and pay close attention to adverse effects. Moreover, the medication education conducted by clinical pharmacists helps patients and their parents capture the correct use of drugs. Through every follow-up, clinical pharmacists provide and reinforce basic education on disease prevention and management of medication administration. Clinical pharmacists reduce medication errors, improve medication utilization, and enhance medication adherence.
Strengths and limitations
Pharmacists are critical members of care teams, and the inclusion of pharmacists in direct patient care improves control of chronic conditions (38). This study has significant implications for the route of pharmaceutical services in the clinical setting of transplantation for pediatric recipients. It may help others develop a more complete clinical pathway on the patient-care based on therapeutic care pathways for the pediatric recipients. Several limitations need to be noted regarding the present study. One of them is that the sample size is relatively small. It is mainly because the groups of pediatric kidney transplantation originating from infants and young children have a relatively small sample size. That’s why we could not carry a trial in which some patients undergo pharmaceutical services and some don't, and compare their current outcomes. Moreover, the patient-care based on pharmaceutical services aimed to individualize the drug therapy for each pediatric patient, to ensure their good clinical outcome. Therefore, all pediatric patients were followed by our patient-centered pharmaceutical services based on therapeutic care pathways. The second limitation is that we lack long-term follow-up data. As missing to follow-up is an inherent problem in the longitudinal study, this study might also suffer from this problem. Thirdly is the retrospective and observational nature of the study, and it is limited to practice at one center. Further research should be undertaken to explore how to choose a suitable individualized dosing regimen for pediatric kidney transplantation patients, and how to achieve precision surveillance for adverse events.
Conclusions
The three-step-protocol of pharmaceutical services strategy in this study report is based on guidelines for transplant perioperative treatment in China and the United States. We recommend this management model for the perioperative patent-care in pediatric patients who had received transplants, especially those who received their kidney from infants and young children. This study strengthens the idea that clinical pharmacists can enhance the quality of patient-centered care based on three-step-protocol of pharmaceutical services for pediatric patients receiving kidney transplantation. Further studies are required to better understand the roles that clinical pharmacists play in the immunosuppression, medical management infection prevention and treatment pre- and post-transplantation, with a multi-disciplinary team approach and of course the multi-center randomized controlled trials that have allowed for meaningful outcome analysis.
Acknowledgments
Funding: This study was supported by the Innovative Clinical Research Funding Project of Changzheng Hospital (No. 2020YLCYJ-Y25), and 2020 Shanghai “New Star of Medicine” and Jin-Zi-Ta Talent Projects (Nos. 0806 and 1016).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-515/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-515/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-515/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-515/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the Changzheng Hospital, Naval Medical University (No. 2019SL021). Informed consent was obtained from each patient or their family members. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rees L. Long-term outcome after renal transplantation in childhood. Pediatr Nephrol 2009;24:475-84. [Crossref] [PubMed]
- Shen Q, Fang X, Man X, et al. Pediatric kidney transplantation in China: an analysis from the IPNA Global Kidney Replacement Therapy Registry. Pediatr Nephrol 2021;36:685-92. [Crossref] [PubMed]
- Su X, Shang W, Liu L, et al. Transplantation of a single kidney from pediatric donors less than 10 kg to children with poor access to transplantation: a two-year outcome analysis. BMC Nephrol 2020;21:250. [Crossref] [PubMed]
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.11 Paediatrics (specific problems). Nephrol Dial Transplant 2002;17:55-8. [PubMed]
- Veronese F, Graziola F, Cammarata E, et al. The Diagnostic-Therapeutic Care Pathway in Psoriasis: Towards ISO 9001:2015 Certification. Medicina (Kaunas) 2020;56:253. [Crossref] [PubMed]
- Balani SS, Jensen CJ, Kouri AM, et al. Induction and maintenance immunosuppression in pediatric kidney transplantation-Advances and controversies. Pediatr Transplant 2021;25:e14077. [Crossref] [PubMed]
- Höcker B, Tönshoff B. Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option? Paediatr Drugs 2011;13:49-69. [Crossref] [PubMed]
- Shendi AM, Wallis G, Painter H, et al. Epidemiology and impact of bloodstream infections among kidney transplant recipients: A retrospective single-center experience. Transpl Infect Dis 2018; [Crossref] [PubMed]
- Metz DK, Holford N, Kausman JY, et al. Optimizing Mycophenolic Acid Exposure in Kidney Transplant Recipients: Time for Target Concentration Intervention. Transplantation 2019;103:2012-30. [Crossref] [PubMed]
- Jacobi J, Ray S, Danelich I, et al. Impact of the Pharmacy Practice Model Initiative on Clinical Pharmacy Specialist Practice. Pharmacotherapy 2016;36:e40-9. [Crossref] [PubMed]
- State Administration of Traditional Chinese Medicine. Interim provisions on the administration of pharmaceutical. Affairs in Medical Institutions 2002; Available online: http://fjs.satcm.gov.cn/gongzuodongtai/2018-03-24/2297.html. Accessed 12 Jan 2022 (in Chinese)
- Qin SB, Zhang XY, Fu Y, et al. The impact of the clinical pharmacist-led interventions in China: A systematic review and Meta-Analysis. Int J Clin Pharm 2020;42:366-77. [Crossref] [PubMed]
- Guo Q, Guo H, Song J, et al. The role of clinical pharmacist trainees in medication reconciliation process at hospital admission. Int J Clin Pharm 2020;42:796-804. [Crossref] [PubMed]
- Xu XF, Feng YT, Tian YF, et al. Pharmaceutical Care in Kidney Transplant Recipients: Behavioral and Physiologic Outcomes at 12 Months. Transplant Proc 2018;50:2451-6. [Crossref] [PubMed]
- Covvey JR, Mancl EE. Pharmaceutical care in transplantation: current challenges and future opportunities. Nanomedicine (Lond) 2019;14:2651-8. [Crossref] [PubMed]
- Williams GM, Lee HM, Hume DM. Renal transplants in children. Transplant Proc 1969;1:262-6. [PubMed]
- LaPlante MP, Kaufman JJ, Goldman R, et al. Kidney transplantation in children. Pediatrics 1970;46:665-77. [Crossref] [PubMed]
- Hebert SA, Swinford RD, Hall DR, et al. Special Considerations in Pediatric Kidney Transplantation. Adv Chronic Kidney Dis 2017;24:398-404. [Crossref] [PubMed]
- Holmberg C, Jalanko H. Long-term effects of paediatric kidney transplantation. Nat Rev Nephrol 2016;12:301-11. [Crossref] [PubMed]
- Hill P, Cross NB, Barnett AN, et al. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev 2017;1:CD004759. [Crossref] [PubMed]
- Bunnapradist S, Takemoto SK. Multivariate analysis of antibody induction therapy and their associated outcomes in deceased donor transplants. Transplant Proc 2005;37:889-91. [Crossref] [PubMed]
- Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006;355:1967-77. [Crossref] [PubMed]
- Tejani A, Emmett L. Acute and chronic rejection. Semin Nephrol 2001;21:498-507. [Crossref] [PubMed]
- Allison TL. Immunosuppressive Therapy in Transplantation. Nurs Clin North Am 2016;51:107-20. [Crossref] [PubMed]
- Trompeter R, Filler G, Webb NJ, et al. Randomized trial of tacrolimus versus cyclosporin microemulsion in renal transplantation. Pediatr Nephrol 2002;17:141-9. [Crossref] [PubMed]
- Chua A, Cramer C, Moudgil A, et al. Kidney transplant practice patterns and outcome benchmarks over 30 years: The 2018 report of the NAPRTCS. Pediatr Transplant 2019;23:e13597. [Crossref] [PubMed]
- Nehus EJ, Liu C, Lu B, et al. Graft survival of pediatric kidney transplant recipients selected for de novo steroid avoidance-a propensity score-matched study. Nephrol Dial Transplant 2017;32:1424-31. [Crossref] [PubMed]
- Sarwal MM, Ettenger RB, Dharnidharka V, et al. Complete steroid avoidance is effective and safe in children with renal transplants: a multicenter randomized trial with three-year follow-up. Am J Transplant 2012;12:2719-29. [Crossref] [PubMed]
- Nehus E, Goebel J, Abraham E. Outcomes of steroid-avoidance protocols in pediatric kidney transplant recipients. Am J Transplant 2012;12:3441-8. [Crossref] [PubMed]
- Birdwell KA, Decker B, Barbarino JM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther 2015;98:19-24. [Crossref] [PubMed]
- Hamadeh IS, Zhang Q, Steuerwald N, et al. Effect of CYP3A4, CYP3A5, and ABCB1 Polymorphisms on Intravenous Tacrolimus Exposure and Adverse Events in Adult Allogeneic Stem Cell Transplant Patients. Biol Blood Marrow Transplant 2019;25:656-63. [Crossref] [PubMed]
- Smith JM, Stablein D, Singh A, et al. Decreased risk of renal allograft thrombosis associated with interleukin-2 receptor antagonists: a report of the NAPRTCS. Am J Transplant 2006;6:585-8. [Crossref] [PubMed]
- Singh A, Stablein D, Tejani A. Risk factors for vascular thrombosis in pediatric renal transplantation: a special report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation 1997;63:1263-7. [Crossref] [PubMed]
- Esfandiar N, Otukesh H, Sharifian M, et al. Protective effect of heparin and aspirin against vascular thrombosis in pediatric kidney transplants. Iran J Kidney Dis 2012;6:141-5. [PubMed]
- Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e737S-801S.
- Verghese PS. Pediatric kidney transplantation: a historical review. Pediatr Res 2017;81:259-64. [Crossref] [PubMed]
- Säemann M, Hörl WH. Urinary tract infection in renal transplant recipients. Eur J Clin Invest 2008;38:58-65. [Crossref] [PubMed]
- Carter BL. Primary Care Physician-Pharmacist Collaborative Care Model: Strategies for Implementation. Pharmacotherapy 2016;36:363-73. [Crossref] [PubMed]


