
A systematic review and meta-analysis of pulmonary surfactant combined with budesonide in the treatment of neonatal respiratory distress syndrome
Instruction
Neonatal respiratory distress syndrome (NRDS), also known as neonatal hyaline membrane disease, is mainly caused by the lack of pulmonary surfactant (PS), leading to progressive alveolar collapse. Progressive dyspnea, groaning, cyanosis, and three-concave inhalation signs appear within 4 to 12 h after birth. In severe cases, respiratory failure may occur (1). The morbidity is related to gestational age. The smaller the gestational age, the higher the morbidity, and the lower the weight, the higher the mortality rate. Imaging examination is an important auxiliary method for the diagnosis and prognosis of NRDS, and it is of great significance for clinical treatment guidance to reduce neonatal mortality by means of imaging examination for the early identification and diagnosis of NRDS in a timely manner and effective treatment measures. Ultrasonography is widely used in clinical practice with the advantages of good safety, strong repeatability and easy operation (2). In recent years, lung ultrasound diagnosis and treatment technology has progressed with the continuous development of ultrasound technology, which can semi-quantitatively assess the ventilation status and lung water content of the lungs, so as to make judgments on the degree and nature of lung lesions, and become an important auxiliary diagnosis and treatment method for respiratory critical diseases (3). At present, the main methods for the treatment of NRDS are basic life support combined with mechanical ventilation and PS replacement therapy. However, there are still long periods of invasive mechanical ventilation, a high incidence of bronchopulmonary dysplasia (BPD), and long hospital stays. Glucocorticoids (GCs) have anti-inflammatory and anti-allergic effects, can reduce bronchial and pulmonary edema, promote the production of antioxidant enzymes and PS, and help improve lung function, thereby reducing the incidence of BPD (4). However, intravenous GCs have relatively large side effects and many complications, and intravenous administration is no longer recommended (5). Budesonide is a GC with strong local anti-inflammatory effects and fewer systemic adverse reactions. According to pharmacokinetic data, approximately 5–10% of budesonide may remain in the lungs after 1 week, and absorbed budesonide in the circulation is rapidly metabolized in the liver to 16-α-hydroxyprednisolone, and its GC activity is low. The half-life of oral budesonide through blood clearance is approximately 4 h (6), which is shorter than the half-life of most GCs. Various animal studies have shown that intratracheal administration of surfactants and GCs can improve lung function (7-10). Direct intratracheal administration of budesonide alone has not been proven effective (11). A previous study showed that inhalation of budesonide can reduce the incidence of death or BPD in low-birth-weight infants (12). Preliminary studies have shown that after instilling pulmonary surfactant and budesonide into the trachea, more than 80% of budesonide may stay in the lungs for up to 8 h (6). Study has shown that the combined use of budesonide and lung surfactants can increase the solubility of budesonide in the lungs, thereby increasing the efficiency of budesonide use (13). Budesonide is not decomposed by lung cells but is extensively combined with fatty acids to form budesonide esters (14). This reversible combination may prolong its local anti-inflammatory effect in the lungs, which may explain why budesonide can be effective for several days even with only one or two doses (6). This article aims to evaluate the safety and effectiveness of pulmonary surfactant combined with budesonide in the treatment of neonatal respiratory distress syndrome through a meta-analysis, which can provide an evidence-based strategy for clinical diagnosis and treatment. Unlike previous study of basic life support combined with mechanical ventilation and PS replacement therapy (2), the analysis of pulmonary surfactant combined with budesonide, a minimally traumatic therapy, may provide a new basis for future clinical treatments and reduce neonatal morbidity and mortality. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-8/rc).
Methods
Search strategy
Relevant studies published until May 30th, 2021 pertaining to the treatment of pulmonary surfactant combined with budesonide in NRDS were searched in online databases such as Wanfang, Weipu, CNKI, PubMed, Embase, and Cochrane Library. In the current study, articles were identified by utilizing the following Chinese keywords: “Neonatal respiratory distress syndrome, Neonatal hyaline membrane disease”, “Pulmonary surfactant, alveolar surfactant”, “Budesonide, glucocorticoid” and English keywords “exogenous surfactant”, “pulmonary surfactant”, “Budesonide”, “hyaline membrane disease of newborn”, “neonatal respiratory distress syndrome”, “neonate respiratory distress syndrome”, and “newborn respiratory distress syndrome”. In PubMed, for example, the search strategy was as follows:
- #1 (exogenous surfactant) OR (pulmonary surfactant)) OR (pulmonary surfactants));
- #2 (hyaline membrane disease of newborn)) OR (neonatal respiratory distress syndrome)) OR (neonate respiratory distress syndrome)) OR (newborn respiratory distress syndrome));
- #3 (Budesonide);
- #1 and #2 and #3.
Study criteria and selection
Publications related to pulmonary surfactant combined with budesonide in the treatment of NRDS were searched.
Studies that met the following criteria were included: (I) randomized controlled trial (RCT); (II) the subjects were premature infants with obvious clinical symptoms or imaging evidence of respiratory distress; (III) the intervention method of the experimental group was PS endotracheal dripping combined with budesonide treatment, and the PS type was animal-derived lung surfactant extract; (IV) the control group was treated with pulmonary surfactant dripping into the trachea, and the PS type was animal-derived pulmonary surfactant extract; (V) at least one primary or secondary outcome measure set out in this paper was reported.
The exclusion criteria were as follows: (I) non-RCT studies; (II) duplicate articles; (III) papers that cannot extract, transform, or retrieve data; (IV) review or systematic evaluation of articles; (V) studies with vague outcome indicators and obvious data errors; (VI) studies irrelevant to the theme or papers that were not open access.
Data extraction and management
Duplicate documents were removed, and the remaining articles were independently screened by two reviewers based on the main content of the abstract. Independent reviewers give suggestions on the article and discussed the acceptability of the article. Independent reviewers standardized the data of the article as follows: (I) first author and publication year; (II) general information; (III) intervention mode; (IV) outcome indicators.
Quality assessment
The quality of RCTs included in the study was assessed using the Cochrane risk of bias tool. The tool includes five items: evaluation of random sequence generation and allocation hiding, blinding of participants and personnel, blinding of result evaluation, and selective reporting bias. Then, studies in each field were divided into three categories: “low risk”, “high risk” and “unclear risk”.
Statistical analysis
All data were analyzed using Review Manager 5.3 software. Cochran Q statistics and I2 statistics were used to assess heterogeneity. Random effects models and fixed effects models (P>0.1 and I2<50%) were used to assess heterogeneity.
Results
Study selection
We retrieved 636 studies from PubMed, Wangfang, and CNKI. After removing duplicate documents, 255 articles were included in the screening of article content; 381 articles were excluded by excluding abstracts or titles that did not meet the requirements. Of the remaining 86, 68 studies that did not fit the type of study and had missing data were excluded. The remaining 10 eligible studies were included in the analysis (Figure 1).
General information
The subjects of this study were premature infants diagnosed with RDS after birth. Among the 10 articles that met the criteria, 8 of them included 977 cases in total. The experimental group (PS and budesonide mixture intratracheal dripping) included 480 cases vs. the control group which included 497 cases. In the other two studies, a total of 200 subjects were studied. The experimental group (100 cases) was treated with tracheal PS and budesonide atomization inhalation, while the control group (100 cases) was treated with tracheal PS only. The basic characteristics of the included literature are shown in Table 1.
Table 1
| Author and publication year | T/C (n) | Gestational weeks | Weight of birth (g) | Intervention | |||||
|---|---|---|---|---|---|---|---|---|---|
| Trial group | control | Trial group | control | Usage and dosage of trial | Usage and dosage of control | ||||
| Yeh (15), 2016 | 131/134 | 26.5±2.2 | 26.8±2.2 | 882±249 | 935±283 | PS (poractant alfa injection 100 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (poractant alfa injection 100 mg/kg) tracheally | ||
| Siqi Chen (16), 2019 | 52/50 | 30.84±1.78 | 31.03±1.96 | 1,556.54±350.44 | 1,589.40±462.71 | PS (poractant alfa injection 100 mg/kg, 150–200 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (poractant alfa injection 150–200 mg/kg) tracheally | ||
| Bo Yang (17), 2021 | 97/101 | 30.6±1.6 | 30.8±1.7 | 1,535±351 | 1,540±340 | PS (phospholipid of bovine 88.2±37.3 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (phospholipid of bovine 88.5±44.6 mg/kg) tracheally | ||
| Jing Pan (18), 2017 | 15/15 | 29.5±1.8 | 30.0±1.7 | 1,260±240 | 1,360±370 | PS (phospholipid of bovine 70 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (phospholipid of bovine 70 mg/kg) tracheally | ||
| Lijing Deng (19), 2018 | 18/28 | <37 | <37 | <1,500 | <1,500 | PS (poractant alfa injection 150 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (poractant alfa injection 150 mg/kg) tracheally | ||
| Jingzhen Su (20), 2019 | 48/50 | 29.68±1.55 | 29.16±1.45 | 1,351.35±337.77 | 1,211.8±267.78 | PS (poractant alfa injection 200 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (poractant alfa injection 200 mg/kg) tracheally | ||
| Yiping Zhou (21), 2019 | 55/55 | 29.37±1.22 | 29.43±1.25 | 1,287.14±209.25 | 1,256.84±204.81 | PS (poractant alfa injection 150 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (poractant alfa injection 150 mg/kg) tracheally | ||
| Lili Ping (22), 2019 | 64/64 | 29.1±1.24 | 28.93±1.2 | 1,264.93±207.12 | 1,260.33±205.87 | PS (poractant alfa injection 150 mg/kg) with budesonide (0.25 mg/kg), tracheally | PS (poractant alfa injection 150 mg/kg) tracheally | ||
| Yazhou Wang (23), 2018 | 72/72 | 31.42±4.27 | 31.51±4.16 | 1,934.54±282.26 | 1,972.54±275.34 | PS (poractant alfa injection 100 mg/kg) tracheally with budesonide (0.25 mg/kg), aerosol inhalation, 3 days | PS (poractant alfa injection 100 mg/kg) tracheally | ||
| Yaoshuang Wang (24), 2019 | 28/28 | 29.51±0.23 | 29.49±0.27 | 1,320±150 | 1,290±210 | PS (phospholipid of bovine 70 mg/kg) tracheally, with budesonide (0.5 mg +2 mL normal saline aerosol inhalation, 3 days | PS (phospholipid of bovine 70 mg/kg) tracheally | ||
PS, pulmonary surfactant.
Quality assessment of included studies
The Cochrane Risk Bias assessment tool was used to evaluate the quality of the studies. All 10 papers were randomly grouped, of which 2 papers described the random grouping methods and allocation concealment specifically, and the remaining 8 only mentioned random grouping but did not describe the specific grouping method. Only 1 paper implemented triple blinding. None of the 10 papers had preset outcome indicators missing. Only 3 articles described dropouts and losses to follow-up. The results of the bias assessment of the included literature are shown in Figure 2.
Results of meta-analysis of outcome indicators
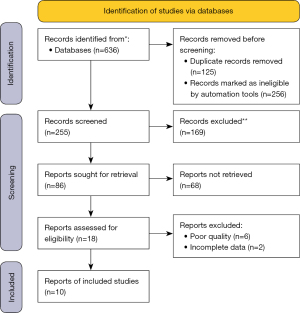
Effect of pulmonary surfactant combined with budesonide on invasive mechanical ventilation time in infants with NRDS
Among the included studies, 9 studies reported the duration of invasive mechanical ventilation, and a total of 912 children were included, including 449 in the experimental group and 463 in the control group. The statistical results showed that P<0.00001, I2=91%, so the random effect model was adopted. Meta-analysis results showed that pulmonary surfactant combined with budesonide in the treatment of NRDS could effectively reduce the duration of invasive mechanical ventilation, with statistical significance between the two groups (OR =−1.72, 95% CI: −2.44 to 1.01, P<0.00001). Subgroup analysis was conducted according to different administration methods. The time of invasive mechanical ventilation in the tracheal dripping subgroup was significantly different from that in the control group, and the combined effect value of the random effect model was OR =−1.30 (95% CI: −1.72 to −0.88, P<0.00001). The duration of invasive mechanical ventilation in the atomized inhalation subgroup was shorter in the experimental group than in the control group, but the difference was not statistically significant, and the combined effect value of the random effect model was (OR =−3.24, 95% CI: −6.60 to 0.13, P=0.06), which is shown in Figure 3.
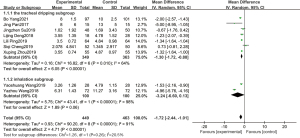
Effect of pulmonary surfactant combined with budesonide on the incidence of BPD in children with NRDS
Among all the included studies, 8 studies reported the incidence of BPD. A total of 1,075 children were included, including 534 in the experimental group and 541 in the control group. The statistical results showed that (P=0.55, I2=0%), so a fixed-effect model was adopted. Meta-analysis results showed that pulmonary surfactant combined with budesonide treatment of NRDS could significantly reduce the incidence of BPD, and there was statistical significance in the incidence of BPD between the experimental group and the control group (OR =0.52, 95% CI: 0.39–0.68, P<0.00001). Subgroup analysis was conducted according to different administration methods, and the incidence of BPD in the tracheal dripping subgroup was significantly different from that in the control group, while the combined effect value of the fixed effect model was OR =0.52, 95% CI: 0.39–0.70, P<0.0001. The incidence of BPD in the atomized inhalation subgroup was lower in the experimental group than in the control group, but the difference was not statistically significant, and the combined effect value in the fixed effect model was OR =0.46, 95% CI: 0.19–1.12, P=0.09. The details are shown in Figure 4.
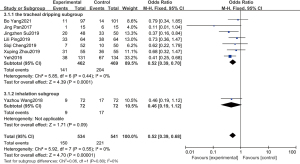
Effect of pulmonary surfactant combined with budesonide on length of hospital stay in infants with NRDS
In all the included studies, 7 studies reported the length of hospital stay, and a total of 738 newborns were included, including 362 in the experimental group and 376 in the control group. The statistical results showed P<0.00001, I2=90%, so a random-effects model was adopted. Meta-analysis showed that pulmonary surfactant combined with budesonide could significantly reduce the length of hospital stay for NRDS (OR =−5.17, 95% CI: −9.35 to −0.99, P=0.02). The duration of hospital stay in the experimental group was shorter than that in the control group, but the difference was not statistically significant. The combined effect value of the random effect model was OR =−4.25 (95% CI: −8.72 to 0.21, P=0.06). The length of hospital stay of the atomization subgroup was significantly different from that of the control group, and the combined effect value of the random effect model was OR =−10.35 (95% CI: −14.18 to −6.52, P<0.00001). The details are shown in Figure 5.
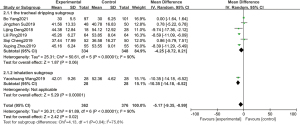
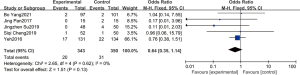
Effect of pulmonary surfactant combined with budesonide on the mortality of infants with NRDS
Five studies reported 693 deaths in total, including 343 in the experimental group and 350 in the control group. The statistical results showed that P=0.62, I2=0%, so the fixed-effect model was adopted. Meta-analysis showed that pulmonary surfactant combined with budesonide could reduce the mortality rate of NRDS, but there was no significant difference. The combined effect value of the fixed effect model was OR =0.64 (95% CI: 0.35–1.14, P=0.13). All five studies were administered intratracheally, and no deaths were reported in the aerosol inhalation study, so a subgroup analysis was not required (Figure 6).
Effect of pulmonary surfactant combined with budesonide on other complications in infants with NRDS
There was no significant difference in the incidence of related complications, such as retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), patent ductus arteriosus (PDA) and septicemia, between the experimental group and the control group (all P values were greater than 0.05). Pulmonary surfactant combined with budesonide does not increase the risk of complications associated with NRDS (Table 2).
Table 2
| Outcome indicators | Articles | Heterogeneity | Experimental group | Control group | OR (95% CI) | P |
|---|---|---|---|---|---|---|
| ROP | (6-8,11-13) | P=0.35, I2=10% | 89/447 | 88/454 | 1.03 (0.73–1.47) | 0.85 |
| NEC | (6-8,11-13) | P=0.79, I2=0% | 41/447 | 37/454 | 1.14 (0.70–1.83) | 0.60 |
| PDA | (6,7,11) | P=0.07, I2=62% | 60/231 | 88/234 | 0.59 (0.28–1.24) | 0.17 |
| Sepsis | (6,8,12,13) | P=0.71, I2=0% | 37/347 | 53/354 | 0.66 (0.42–1.06) | 0.08 |
NRDS, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; OR, odds ratio; CI, confidence interval.
Discussion
Effect of pulmonary surfactant combined with budesonide on invasive mechanical ventilation time, the incidence of BPD, hospital stay and other complications in infants with NRDS
The common cause of NRDS is premature delivery, which can cause heart failure and brain developmental disorders in severe cases. We can quickly and accurately diagnose NRDS by examining the patient’s clinical symptoms, changes in lung X-rays, and lung maturity. Clinically, mechanical ventilation is generally given. However, the lungs of premature babies are not fully developed, and mechanical ventilation will cause varying degrees of damage to the lung tissue structure, leading to a large amount of tissue fiber growth, a decrease in the number of alveoli, and an increase in lung resistance, resulting in a severe decline in lung function. The injured lung is susceptible to bacterial invasion and infection, leading to long-term reliance on ventilators or weaning difficulties, prolonging the hospitalization time of children, increasing the incidence and mortality of BPD, and increasing the risk of infection due to other complications. In this article, a meta-analysis of the results of the included 10 studies showed that the duration of mechanical ventilation and hospitalization of children in the experimental group was shortened, and the incidence of BPD was reduced. This is consistent with the conclusion of Yan that other pulmonary surfactant combined with budesonide can quickly improve the lung function of children with NRDS so that the ventilator can be withdrawn as soon as possible (25). After pulmonary surfactant is injected into the lungs, under the influence of lung surface tension, it quickly disperses on the surface of the alveoli, promoting the expansion of atrophic alveoli, establishing an effective gas exchange platform, reducing the lung resistance of the child, and improving the functional level of the lungs, which shortens the time of mechanical ventilation and the length of hospitalization. In addition, budesonide can fight local inflammation, has good anti-inflammatory and antiviral effects in local lung tissues, and inhibits the occurrence of lung inflammation. During the inhalation process, it cannot be ensured that all the drugs will be delivered to the deep alveoli of the child, which might cause part of the drug to be suspended on the wall of the tracheal tube in granular form, thus reducing the efficacy or causing irritation to the local lung tissue. Pulmonary surface-active substances have the effect of reducing the surface tension of the alveoli and can evenly distribute the drug in the lungs, promoting its absorption of budesonide and therefore playing a dual role in the drug effect, which improves the clinical efficacy. The statistical analysis of the mortality of the two groups showed that the difference between the experimental group and the control group was not statistically significant. The analysis of complications related to neonatal respiratory distress syndrome suggested that the difference between the experimental group and the control group was not statistically significant. A short-term follow-up did not find any GC-related sequelae in the experimental group. In summary, pulmonary surfactant combined with budesonide in the treatment of NRDS will not increase the incidence of death and complications, and its clinical application is relatively safe. Therefore, for patients with suitable conditions in clinical treatment, clinicians can consider alveolar surfactant combined with budesonide to treat neonatal respiratory distress syndrome.
Administration methods of budesonide and their therapeutic effect in children with NRDS
In the 10 studies included in this article, budesonide was an inhaled preparation, and the way budesonide was used was not uniform. Two of the articles used aerosol inhalation, and the other 8 used intratracheal instillation. According to the analysis of different administration methods, the subgroup analysis showed that the tracheal instillation subgroup was significantly better than the control group in reducing the time of mechanical ventilation, while the difference in the nebulization group was not statistically significant in reducing the length of hospitalization. The length of hospital stay in the experimental group was significantly less than that in the control group, while the tracheal instillation subgroup had statistically significant differences in the length of stay compared to the experimental group. The subgroup with tracheal instillation demonstrated statistical significance in the reduction and prevention of the occurrence of BPD, and the results within the subgroups were inconsistent. This phenomenon was related to the small sample size. Yang and other scholars proved through piglet model experiments that the combination of surfactant and budesonide intratracheal instillation can improve the lung histological structure of premature piglets and concluded that the combination of intratracheal corticosteroid surfactant is effective in alleviating lung diseases. Thereafter, they concluded that severe RDS could be treated (26). Cole et al. believes that it is difficult for premature infants to inhale budesonide into the alveoli by aerosols, and the anti-inflammatory effect is easily limited. However, direct intratracheal infusion of PS and budesonide can increase the concentration of budesonide in the alveoli, thereby increasing the anti-inflammatory effect (27). However, Hua et al. believe that aerosol budesonide is more easily absorbed in the lungs, and inhalation can allow the maintenance of a certain drug concentration in the lungs, which is conducive to full absorption and function in the lungs (28). Neonatal respiratory distress is caused by a lack of surfactant, long-term respiratory distress may eventually lead to bronchopulmonary dysplasia. At present, study has shown that budesonide-surfactant endotracheal administration is used to prevent infant BPD (10). Intra-tracheal administration of budesonide-surfactant combination was associated with decreased incidence of BPD alone or composite outcome of death or BPD in very low birth weight (VLBW) infants (29). It is further shown that budesonide-surfactant neonatal long-term respiratory distress has a clear clinical advantage in the prevention of infant BPD, providing an evidence-based basis for the treatment of neonatal respiratory distress. Since there were only two studies in the nebulization inhalation group in this article and the sample size was small, no statistical study between subgroups was carried out. However, compared with the control group, the instillation group was more statistically significant in improving the time of mechanical ventilation and reducing the incidence of BPD in the treatment of neonatal respiratory distress than the non-aerosolized inhalation group. Based on the research in this article, we recommend the use of these treatments in clinical practice.
Limitations of this research
Compared with other studies related to neonatal respiratory diseases, the innovation of this study is to focus on the clinical medication of neonatal distress syndrome, and elaborate on the results of budesonide treatment. Due to the small sample size that fits the included studies, the ethnic group involved in the study was relatively limited (China, the United States), most of the included studies did not describe data blindness, and the reported data may have selectivity bias. In addition, there is no uniform standard for drug dosage and frequency of medication in the current administration method. It is still necessary to further develop multicenter, large-sample and blind clinical experimental studies to provide a medical basis for the comprehensive evaluation of the efficacy and safety of pulmonary surfactant combined with budesonide in the treatment of NRDS.
Acknowledgments
Funding: This research was funded by the Guiding Project of Science and Technology Bureau of Enshi Prefecture, Hubei Province (No. E20200018).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-8/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shao XM, Ye HT, Qiu XS. Practical neonatology. Beijing: People’s Medical Publishing House, 2011.
- Mayo PH, Copetti R, Feller-Kopman D, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med 2019;45:1200-11. [Crossref] [PubMed]
- Mojoli F, Bouhemad B, Mongodi S, et al. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med 2019;199:701-14. [Crossref] [PubMed]
- Davis JM, Bhutani VK, Stefano JL, et al. Changes in pulmonary mechanics following caffeine administration in infants with bronchopulmonary dysplasia. Pediatr Pulmonol 1989;6:49-52. [Crossref] [PubMed]
- Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1980;1:55-9. [Crossref] [PubMed]
- Yeh TF, Lin HC, Chang CH, et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics 2008;121:e1310-8. [Crossref] [PubMed]
- Fajardo C, Levin D, Garcia M, et al. Surfactant versus saline as a vehicle for corticosteroid delivery to the lungs of ventilated rabbits. Pediatr Res 1998;43:542-7. [Crossref] [PubMed]
- Chen CM, Fang CL, Chang CH. Surfactant and corticosteroid effects on lung function in a rat model of acute lung injury. Crit Care Med 2001;29:2169-75. [Crossref] [PubMed]
- Yang CF, Lin CH, Chiou SY, et al. Intratracheal budesonide supplementation in addition to surfactant improves pulmonary outcome in surfactant-depleted newborn piglets. Pediatr Pulmonol 2013;48:151-9. [Crossref] [PubMed]
- Patole SK, Mohan MS, Jog SM, et al. Prophylactic intratracheal budesonide in preterm neonates at risk for chronic lung disease (CLD)—a pilot study. Presented at the Annual Conference of the Perinatal Society of Australia and New Zealand. March 2–3, 2001, Canberra, Australia. No. 1590.
- Lin YJ, Lin HC, Lin CH, et al. Double-blind controlled trial of endotracheal instillation of budesonide in preterm infants with RDS: a preliminary report. PAS 2000;5:24-36.
- Bassler D, Carnielli V, Halliday HL, et al. Early inhaled corticosteroids for the prevention of bronchopulmonary dysplasia in extremely preterm infants: the Neonatal European Study of Inhaled Steroids (NEUROSIS). Arch Dis Child 2014;99:A1. [Crossref]
- Wiedmann TS, Bhatia R, Wattenberg LW. Drug solubilization in lung surfactant. J Control Release 2000;65:43-7. [Crossref] [PubMed]
- Miller-Larsson A, Mattsson H, Hjertberg E, et al. Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos 1998;26:623-30. [PubMed]
- Yeh TF, Chen CM, Wu SY, et al. Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 2016;193:86-95. [Crossref] [PubMed]
- Chen SQ. Clinical study of budesonide combined with pulmonary surfactant in the treatment of neonatal respiratory distress syndrome. Guangzhou: Guangdong Medical University, 2019.
- Yang B, Lei HL, Ren Y, et al. Pulmonary surfactant in combination with intratracheal budesonide instillation for treatment of respiratory distress syndrome in preterm infants: a randomized controlled trial. Chinese Journal of Neonatology 2021;36:33-7.
- Pan J, Chen MW, Ni WQ, et al. Clinical efficacy of pulmonary surfactant combined with budesonide for preventing bronchopulmonary dysplasia in very low birth weight infants. Chinese Journal of Contemporary Pediatrics 2017;19:137-41. [PubMed]
- Deng LJ, Peng HB, Gong XQ. Efficacy of intratracheal instillation of budesonide using surfactant as a vehicle to treatment respiratory distress syndrome of preterm infants. Medical Science Journal of Central South China 2018;46:97-100.
- Su JZ, Yang YL, Yang L, et al. The clinical study of budesonide combined with pulmonary surfactant to prevent bronchopulmonary dysplasia in premature infants. International Journal of Pediatrics 2019;46:61-5.
- Zhou XP, Zhou J, Hu B, et al. Effects of Budesonide Combined with Pulmonary Surfactant on Short-Term Efficacy, Blood Oxygenation Index and Risk of Bronchopulmonary Dysplasia in Premature Infants with Severe Respiratory Distress Syndrome. Journal of Pediatric Pharmacy 2019;25:27-30.
- Ping LL, Zhang SY, Zhai SF. The effect of dual bronchial drug infusion program on the clinical prognosis of preterm infants with severe respiratory distress syndrome. Chinese Journal of Postgraduates of Medicine 2019;42:154-7.
- Wang YZ, Zhang DF. Effect of budesonide inhalation combined with pulmonary surfactant on newborns with RDS. Modern Medical Journal 2018;46:1243-6.
- Wang YS, Yang M, Han P, et al. Effect of Budesonide suspension oxygenation atomization inhalation on prevention and treatment of bronchopulmonary dysplasia in premature infants. Journal of Clinical and Experimental Medicine 2019;19:2121-4.
- Yan LB, Han SP, Chu XB, et al. Effect of Using Pulmonary Surfactant Mixed with Budesonide on Pulmonary Function in Very Low Birth Weight Premature with Acute Respiratory Distress Syndrome. Journal of Applied Clinical Pediatrics 2011;26:1400-2.
- Yang CF, Jeng MJ, Soong WJ, et al. Acute pathophysiological effects of intratracheal instillation of budesonide and exogenous surfactant in a neonatal surfactant-depleted piglet model. Pediatr Neonatol 2010;51:219-26. [Crossref] [PubMed]
- Cole CH, Colton T, Shah BL, et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. N Engl J Med 1999;340:1005-10. [Crossref] [PubMed]
- Ke H, Li ZK, Yu XP, et al. Efficacy of different preparations of budesonide combined with pulmonary surfactant in the treatment of neonatal respiratory distress syndrome: a comparative analysis. Chinese Journal of Contemporary Pediatrics 2016;18:400-4. [PubMed]
- Venkataraman R, Kamaluddeen M, Hasan SU, et al. Intratracheal Administration of Budesonide-Surfactant in Prevention of Bronchopulmonary Dysplasia in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Pediatr Pulmonol 2017;52:968-75. [Crossref] [PubMed]



