
Ultrasound-guided erector spinae plane block improve opioid-sparing perioperative analgesia in pediatric patients undergoing thoracoscopic lung lesion resection: a prospective randomized controlled trial
Introduction
Severe pain may occur after pediatric thoracic surgery in cases such as skin incision creation, rib traction, the placement of drains, and in other cases, such as rib nerve injury. Such pain is highly unfavorable to the recovery of pediatric patients as it may lead to reduced cough strength for clearing secretions, decreased functional residual capacity, and pulmonary complications such as atelectasis and pneumonia (1). Although minimally invasive video-assisted thoracoscopic surgery (VATS) can reduce surgical trauma, alleviate postoperative pain, and decrease the incidence of pulmonary complications, children can still experience severe pain in the first few hours after surgery (2,3).
In recent years, the value of regional blocks in multimodal pain management has increasingly been recognized. The use of these techniques may lower the doses of opioids prescribed for each patient, producing the same analgesic effects while reducing adverse effects such as nausea, vomiting, pruritus, and urinary retention (4), thereby improving postoperative comfort and accelerating postoperative recovery. Thus, it offers anesthetic technical support for enhanced recovery after surgery (ERAS). Previously, the commonly used regional block techniques for perioperative analgesia in thoracic surgery included thoracic segmental epidural block and thoracic paravertebral block. However, these methods were technically demanding and had the potential risk of serious complications, including epidural hematoma, total spinal anesthesia, nerve injury, pneumothorax, hypotension, and infection (5-7). With advances in fascial plane blocks, regional anesthesia and analgesia have made great progress in recent years. Among the many advances, the erector spinae plane block (ESPB) was first described in 2016 as a regional block for acute thoracic pain and neuropathic pain (8). The past few years have witnessed its successful application in perioperative analgesia for a variety of thoracic and abdominal surgeries in adult patients. ESPB is simple to administer, and its safety has also been well established as the site of the injection is far from the spinal cord, pleura, and other vital organs and tissues (9,10).
While the role of ESPB in adults has been extensively investigated, few studies have been carried out in pediatric patients. In addition, most published articles were case reports, and there was a lack of randomized controlled studies with larger sample sizes. Erector spinae plane block is an inter-fascial plane block whereby local anesthetic (LA) is injected beneath the erector spinae muscles to achieve multimetameric analgesia for pediatric thoracic, cardiac, or abdominal surgery. LA appears to be penetrate anteriorly to enter the thoracic paravertebral space where it blocks both the ventral and dorsal rami of spinal nerves as well as the rami communicantes that convey sympathetic fibers for its analgesic effects. We hypothesized that in children undergoing thoracoscopic resection of pulmonary lesions, general anesthesia (GA) combined with ESPB at the T4 level might reduce the quantity of perioperative opioids required and improve early postoperative analgesia compared with the use of GA alone. In our current study, we compared the administration of GA + ESPB at the T4 level with using GA alone in children undergoing VATS. Our study parameters included the doses of intraoperative remifentanil and postoperative sufentanil, the children’s face, legs, activity, cry, consolability (FLACC) scores at rest, the time to first rescue analgesia, and parental satisfaction with analgesia in a randomized controlled trial. In addition, we tried to demonstrate that a single preoperative dose of ESPB at the T4 level could enhance intraoperative and postoperative analgesia in children. We present the following article in accordance with the CONSORT reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-118/rc).
Methods
General data
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tianjin Children’s Hospital (No. L2022-02). All parents (or legal guardians) of the children enrolled in this trial were informed about the trial and the possible adverse outcomes and signed informed consent forms. The sample size was calculated by using G*Power software prior to the start of the main study. For this purpose, 10 patients were enrolled in the preceding pilot study (n=5 in each group), and the sample size was calculated as 26 children per group for obtaining statistically significant values [with significance level α=0.05, type II error (value of β) =0.2, means of consumptions of sufentanil in GA + ESPB and GA 0.42, 0.3, SD of consumptions of sufentanil 0.15]. Given the possibility of dropouts, we enrolled 30 children in each group. We selected children aged 1 to 3 years, with American Society of Anesthesiologists (ASA) grades I to II, and a BMI of 15 to 20 kg/m2, who were scheduled to undergo a lung lesion resection (lung lobectomy or segmentectomy) via VATS in the Tianjin Children’s Hospital from March 2021 to March 2022.
The exclusion criteria for the trial were as follows: (I) patient with a history of allergy to amide local anesthetics; (II) patient with abnormal liver/kidney function; (III) patient with severe spinal deformities; and (IV) patient with skin damage or infection at the proposed puncture site. Children were classified as dropouts in the following cases: (I) they converted to an open procedure during surgery; (II) they experienced severe hypotension or showed signs of arrhythmia after anesthesia; (III) their surgery lasted more than 3 hours; (IV) they required mechanical ventilation after surgery.
The children and their parents, the observers involved in postoperative pain scoring, and the statistical analysts were blinded to this study. The children were randomly grouped by the study designer using the random number table method in SAS software. The grouping numbers were kept in opaque, sealed envelopes. During the anesthesia visit the day before surgery, an anesthesiologist gave each participant an envelope, which was brought into the operating room on the operation day. The responsible anesthesiologist opened the envelope and performed the anesthesia according to the grouping result within. In this two-parallel study, participants were randomly divided 1:1 into two groups: a GA + ESPB group and a GA group. The GA group received GA alone, and the GA + ESPB group received ESPB at the level of the T4 transverse process in addition to GA.
Anesthesia
Children were fasted for 6 hours for solids and for 2 hours for clear fluids before the procedure. Atropine (0.01 mg/kg) was administered intramuscularly 30 minutes before surgery. After the patient entered the operating room, their pulse oxygen saturation (SpO2), non-invasive blood pressure (NIBP), and heart rate (HR) were routinely monitored. Anesthesia was induced by inhalation of a gas mixture of 8% sevoflurane in 100% oxygen. Subsequently, midazolam (0.2 mg/kg), sufentanil (0.3 μg/kg), and atracurium (0.4 mg/kg) were intravenously injected. After a stable anesthetic level was obtained, the tracheal tube was inserted orally. When the position of the tube was determined to be correct by auscultation, a bronchial blocker was advanced via the tracheal tube under the guidance of fiberoptic bronchoscopy. If the patient was younger than 2 years, the internal diameter (ID) of the selected tracheal tube was below 5.0 mm; in such cases, the bronchial blocker was first placed through the mouth under clear vision, and then the tracheal tube was advanced, so that the bronchial blocker was located on the outside of the tracheal tube. The electronic bronchoscope (ENF-P20, Seesheen, Zhuhai, China) used had an external diameter of 2.8 mm, and the tracheal tube was a single-use general-type uncuffed tracheal tube (Sujia Medical Equipment Co. Ltd., Jiaxing, Zhejiang, China). After successful positioning and fixation of the tracheal tube and bronchial blocker, the radial artery and internal jugular vein were punctured and cannulated. The surgical procedure was then performed in each group. In the GA group, the operation was started immediately after the vital signs became stable; in the GA + ESPB group, a unilateral ESPB was administered in addition to the GA, and the operation was started after the block was successful.
In both groups, anesthesia was maintained using propofol (3 to 6 mg/kg·h) and remifentanil (0.2 to 0.5 μg/kg·min) during the surgery. Doses were adjusted according to the changes in heart rate and blood pressure (the heart rate and blood pressure of the children were maintained within 20% of the preoperative base values), and additional atracurium (0.2 mg/kg) was applied intermittently as needed. During intraoperative one-lung ventilation, the tidal volume was selected at 5 mL/kg, the respiratory rate was 20 to 28 breaths/min, the ETCO2 was maintained at 35 to 50 mmHg, and the peak airway pressure was below 30 cmH2O. Sufentanil (0.2 μg/kg) was injected intravenously 30 minutes before the end of the operation, and the children were extubated in the operating room after the surgery. The conditions for extubating were as follows: (I) patient was awake; (II) spontaneous respiration was restored with a frequency of >12 breaths/min; (III) patient had stable hemodynamics; and (IV) patient had restored protective reflexes. The patients were admitted into the pediatric intensive care unit (PICU) after their vital signs became stable.
The postoperative analgesic regimen in both groups was as follows: as a component of multimodal analgesia, acetaminophen (15 mg/kg) was orally administered every 6 hours, starting on the night after surgery. If children had poor postoperative pain control (with a FLACC score of >4), sufentanil (0.05–0.1 μg/kg) was intravenously administered as rescue analgesia.
ESPB
In this study, we utilized the Philips IU-22 ultrasound system with an L12-5 linear array ultrasound probe transducer. The patients were asked to assume a lateral position on the unaffected side. After disinfection of the skin at the scheduled puncture site, ultrasound-guided ESPB was administered to all patients on the affected side. The block site was selected at the transverse process of the fourth thoracic vertebra on the affected side. The ultrasound probe was placed along the longitudinal axis of the body, 1 to 2 cm away from the spinal spinous process. When the transverse process was accurately localized by ultrasound, a nerve block needle was inserted in-plane in the caudal-to-cephalad direction. After the needle tip reached the surface of the transverse thoracic process, 1 to 2 mL of saline was injected, and 0.25% levobupivacaine (0.5 mL/kg) was injected after the erector spinae muscles were elevated by the saline (Figure 1). After the successful block, the operation was started immediately after vital signs became stable.
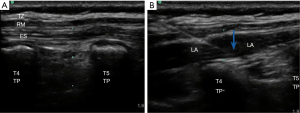
Main measurements
The primary outcome was the amount of postoperative sufentanil administered in both groups, while the secondary outcomes were the postoperative FLACC score and the amount of intraoperative remifentanil. The FLACC score was assessed at 0, 1, 3, 6, 12, 18, and 24 hours after admission to the post-anesthesia care unit (PACU) during rest. The recording and scoring were done by an experienced anesthesiologist who was blind to the grouping status and anesthesia method, and the validity of this pain assessment tool and its cut-off values have been reported to guide the interventional treatment of analgesic drugs (11). The time to first rescue analgesia after surgery, length of hospital stay, and parental satisfaction with postoperative pain management were also recorded. Parental satisfaction was recorded on a numerical scale ranging from 1, representing the greatest dissatisfaction, to the highest possible satisfaction level of 10. Any possible adverse events such as pneumothorax, local tissue infection, nausea, vomiting, skin pruritus, and chills were also recorded.
Statistical analysis
The statistical analysis was completed using the SPSS 17.0 software package with measurement data expressed as mean ± standard deviation and count data expressed as the number of cases and ratio (n, %). Normality was checked using the Shapiro-Wilk test, and the comparisons of the normally distributed measurement data were based on a t-test. The count data were compared using the chi-square test. A P value <0.05 was considered statistically significant. Group, time, and group-time interactions were evaluated using repeated-measures analysis of variance (ANOVA), and further multiple comparisons were performed using a least significant difference (LSD) t-test.
Results
The recruitment took place between March 2021 and March 2022. Sixty-five pediatric patients were evaluated for eligibility, two patients’ parents declined to participate and three patients did not match the inclusion criteria. Sixty children were enrolled and randomly assigned to two groups of 30 patients each. The follow-up time was 24 hours after surgery, all the enrolled children successfully completed the trial and entered the final analysis (Figure 2).
There were no statistically significant differences in age, gender, body mass index (BMI), ASA grade, operative time, and length of hospital stay between the GA + ESPB and GA groups (all P>0.05) (Table 1). In the GA + ESPB group, the amounts of postoperative sufentanil and intraoperative remifentanil administered were 0.26±0.11 and 61.73±6.42 μg/kg, respectively, both of which were lower than the amounts given to the GA group (0.47±0.08 and 88.30±13.33 μg/kg, respectively), mean difference (95% CI): −0.21 (−0.27 to −0.17) and −26.57 (−31.98 to −21.17), respectively. The time to first rescue analgesia in the GA + ESPB group was 5.15±1.41 hours, which was significantly longer than that in the GA group (2.79±0.85 hours), mean difference (95% CI): 2.37 (1.77 to 2.97). The parental satisfaction score for postoperative analgesia was 7.23±1.19 points in the GA + ESPB group, which was significantly higher than in the GA group score of 4.77±1.43 points, mean difference (95% CI): 2.47(1.79 to 3.15) (all P<0.001) (Table 2). The effects of group, time, and group*time on the children’s FLACC scores were also statistically significant (F values: 260.789, 79.882, and 13.387, respectively; P<0.001). Multiple comparisons showed that FLACC scores were lower in the GA + ESPB group than in the GA group at 1 to 24 hours postoperatively (P=0.023 at 1 h, and P<0.001 at 3 h, 6 h, 12 h, 18 h, 24 h), but not upon immediate PACU entry (P=0.189 at 0 h) (Figure 3). In the GA group, 11 children had postoperative nausea, and 9 had vomiting, while in GA + ESPB group, only 4 children experienced nausea, and 2 had vomiting, the difference was statistically significant (P=0.037 and P=0.020). Skin pruritus was noted in 3 members of the GA + ESPB group and 7 members of the GA group, but the difference was not statistically significant (P=0.17). No pneumothorax, local tissue infection, or postoperative chills were noted in either group (Table 3).
Table 1
| Items | GA + ESPB group | GA group | F or χ2 |
|---|---|---|---|
| Gender (male/female) | 12/18 | 10/20 | 0.287 |
| Age (years) | 1.89±0.62 | 2.02±0.39 | 4.78 |
| ASA grade (I/II) | 22/8 | 20/10 | 0.317 |
| BMI (kg/m2) | 16.37±0.90 | 16.71±1.23 | 3.57 |
| Operative time (min) | 120.03±9.78 | 118.20±12.94 | 2.214 |
| Length of hospital stay (days) | 13.33±1.71 | 13.60±1.57 | 0.085 |
Data in the table are expressed as cases or ; all P>0.05. GA, general anesthesia; ESPB, erector spinae plane block; ASA, American Society of Anesthesiology; BMI, body mass index; F, F statistic.
Table 2
| Items | GA + ESPB group | GA group | Mean difference (95% CI) | F |
|---|---|---|---|---|
| Amount of sufentanil (μg/kg) | 0.26±0.11 | 0.47±0.08 | −0.21 (−0.27 to −0.17) | 3.07** |
| Amount of remifentanil (μg/kg) | 61.73±6.42 | 88.30±13.33 | −26.57 (−31.98 to −21.17) | 10.45** |
| Time to first rescue analgesia (h) | 5.15±1.41 | 2.79±0.85 | 2.37 (1.77 to 2.97) | 5.60** |
| Parental satisfaction (points) | 7.23±1.19 | 4.77±1.43 | 2.47 (1.79 to 3.15) | 1.20** |
Data in the table are expressed as . **, P<0.01. GA, general anesthesia; ESPB, erector spinae plane block; 95% CI, 95% confidence interval; F, F statistic.
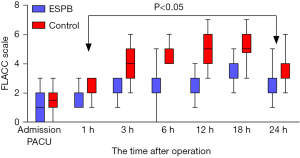
Table 3
| Items | GA + ESPB group (n=30) (Y/N) | GA group (n=30) (Y/N) | χ2 |
|---|---|---|---|
| Nausea | 4/26 | 11/19 | 4.356* |
| Vomiting | 2/28 | 9/21 | 5.455* |
| Pruritus | 3/27 | 7/23 | 1.920 |
| Pneumothorax | 0 | 0 | – |
| Local tissue infection | 0 | 0 | – |
| Chills | 0 | 0 | – |
Data in the table are expressed as cases. *, P<0.05. GA, general anesthesia; ESPB, erector spinae plane block; Y/N, yes/no.
Discussion
As shown in our current study, compared with GA alone, preoperative ESPB could alleviate postoperative pain in children undergoing a lung lesion resection via VATS. It could also prolong the time to first rescue analgesia and reduce the need for postoperative opioids, thereby reducing the side effects associated with opioid use and increasing parental satisfaction with postoperative pain management. Furthermore, it helped to lower the amount of intraoperative analgesia.
In the past, opioids were the primary analgesics for pain management after thoracic surgery. Although the use of opioids can alleviate pain, the side effects (e.g., nausea, vomiting, and possible respiratory depression or drug addiction) can adversely affect postoperative recovery. Currently, many national and regional anesthesia guidelines advocate opioid-free anesthesia (OFA) protocols (12,13). However, if acute postoperative pain is not effectively controlled, some patients may develop chronic pain (14). In the choice of analgesic regimen, combinations with regional block techniques seem to be more reasonable than the application of analgesic drugs alone that can cause systemic effects. At present, epidural analgesia and thoracic paravertebral nerve block are the most recognized regional block techniques, with promising efficacy; however, they are technically challenging for pediatric patients, and their use is limited by the potential for serious complications, such as pneumothorax and total spinal anesthesia. In contrast, ultrasound-guided ESPB is a safer analgesic technique and is technically simpler than the above 2 modalities. Therefore, many authors have proposed that it could be used as a component of multimodal analgesia to accelerate postoperative recovery (15-17). Although ultrasound-guided ESPB is mainly used for adult patients, some pediatric indications have been reported (18-20). Our current study demonstrated the safety of ultrasound-guided ESPB. The incidence of nausea and vomiting in GA + ESPB group was significantly lower than that in the GA group, and no pneumothorax, local tissue infection, or chills were noted.
During ESPB, a local anesthetic drug is injected into the area between the transverse process and the deep surface of the erector spinae muscle; after osmosis, the local anesthetic can reach the thoracic paravertebral space and epidural cavity and block the communicating branches of the spinal nerve root containing sympathetic nerves, thus producing analgesic effects on both the body and the viscera (8,21). However, the mechanisms behind the action of ESPB remain controversial. Ivanusic et al. performed an autopsy study on 10 fresh cadavers, injecting 20 mL of 0.25% methylene blue dye bilaterally into the plane between the fifth thoracic transverse processes and erector spinae muscles. After 30 minutes, the dye had spread up and down to the T1 to T12 levels and laterally to the lateral edge of the erector spinae muscle. However, the ventral rami were stained by the dye in only 1 injection, and in only 2 injections did the dye track posteriorly through the costotransverse foramen to the dorsal root ganglion (22). In contrast, Schoenfeldt et al. argued that increasing the volume of the injection to 30 ml would allow for a wider infiltration of the drug. In fact, they observed that the drug could break through the transverse costal foramen to reach the paravertebral and intercostal spaces when 20 mL of the drug was administered (23). Schwartzmann et al. performed an MRI study on 6 adult patients, injecting 30 to 35 mL of local anesthetic containing a contrast agent into the patients at the T10 level. Similarly, they observed diffusion of the drug into the paravertebral and intercostal spaces and even into the epidural space in 2 patients (24). We believe that one possible reason for these differences is that the resistance to diffusion of the drug solution comes mainly from the supra-transverse and inter-transverse ligaments, whereas the permeability of each fascia was greatly reduced in the cadaveric study due to protein denaturation. In terms of the dynamics of diffusion, both the increased pressure of the drug itself due to an increase in the volume of injection and the suctioning effect of negative intrathoracic pressure during patient respiration had positive effects. We believe that the diffusion of the drug may be easier in pediatric patients. This is because fascial tissue is thinner in children than in adults, which results in less resistance to drug spread. Govender et al. injected 0.5 and 0.2 mL of methylene blue dye into the vertebral levels T5 and T8 in cadavers weighing 1.6 and 0.6 kg, respectively. While craniocaudal spread was noted at vertebral levels, the methylene blue spread was also found in the paravertebral, epidural, and intercostal spaces, staining both the dorsal and ventral rami of the spinal nerves (25). These findings were consistent with our hypothesis.
Although the exact mechanism of ESPB is not fully understood, its role in pediatric settings is promising (19,26,27). In the present study, the GA + ESPB group required significantly less postoperative sufentanil than the GA group and had a significantly longer time to first rescue analgesia, suggesting that a single preoperative ESPB can dramatically improve postoperative pain management in children, as proposed by Kaushal et al. (28). In addition, the FLACC scores were lower in the GA + ESPB group than in the GA group at 1 to 24 hours postoperatively, though not at immediate PACU entry, which more directly confirmed the analgesic effect of this technique. There was no statistical difference in FLACC scores between the two groups immediately after entry into the PACU, which may have been due to the residual analgesic effect of the sufentanil (0.2 μg/kg, used to prevent possible nociceptive sensitization due to continuous intravenous infusion of remifentanil) administered 30 minutes before the end of the procedure.
All the participants were children who underwent a lung lesion resection via VATS, which might minimize the impacts of confounding factors on our findings.
Our study had some limitations. First, the sample size was relatively small. Second, because the children were too young to cooperate, ESPB had to be done in addition to GA, which prevented the assessment of the sensory block and meant that we could not perform an objective evaluation of the block range. Finally, we only observed the analgesic effect within 24 hours after surgery, and the long-term effects of this regional block technique need to be further explored.
Conclusions
This study has shown that a single preoperative ESPB reduced the consumptions of opioids for perioperative analgesia in children undergoing a lung lesion resection via VATS, lower FLACC score, facilitate postoperative recovery and improve parental satisfaction with postoperative pain management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-118/rc
Trial Protocol: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-118/tp
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-118/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-118/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tianjin Children’s Hospital (No. L2022-02). All parents (or legal guardians) of the children enrolled in this trial were informed about the trial and the possible adverse outcomes and signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marshall K, McLaughlin K. Pain Management in Thoracic Surgery. Thorac Surg Clin 2020;30:339-46. [Crossref] [PubMed]
- Feray S, Lubach J, Joshi GP, et al. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2022;77:311-25. [Crossref] [PubMed]
- Steinthorsdottir KJ, Wildgaard L, Hansen HJ, et al. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg 2014;45:959-66. [Crossref] [PubMed]
- Khidr AM, Senturk M, El-Tahan MR. Impact of regional analgesia techniques on the long-term clinical outcomes following thoracic surgery. Saudi J Anaesth 2021;15:335-40. [Crossref] [PubMed]
- Ben Aziz M, Mukhdomi J. Thoracic Paravertebral Block. StatPearls. Treasure Island (FL), 2022.
- Freise H, Van Aken HK. Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth 2011;107:859-68. [Crossref] [PubMed]
- Kussman BD. Commentary: Thoracic epidural anesthesia for pediatric cardiac surgery and enhanced recovery: Still lessons yet to be learned. JTCVS Tech 2022;11:69-70. [Crossref] [PubMed]
- Forero M, Adhikary SD, Lopez H, et al. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med 2016;41:621-7. [Crossref] [PubMed]
- Urits I, Charipova K, Gress K, et al. Expanding Role of the Erector Spinae Plane Block for Postoperative and Chronic Pain Management. Curr Pain Headache Rep 2019;23:71. [Crossref] [PubMed]
- Helander EM, Webb MP, Kendrick J, et al. PECS, serratus plane, erector spinae, and paravertebral blocks: A comprehensive review. Best Pract Res Clin Anaesthesiol 2019;33:573-81. [Crossref] [PubMed]
- Crellin DJ, Harrison D, Santamaria N, et al. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain 2015;156:2132-51. [Crossref] [PubMed]
- Nachiyunde B, Lam L. The efficacy of different modes of analgesia in postoperative pain management and early mobilization in postoperative cardiac surgical patients: A systematic review. Ann Card Anaesth 2018;21:363-70. [Crossref] [PubMed]
- Meyenfeldt EMV, van Nassau F, de Betue CTI, et al. Implementing an enhanced recovery after thoracic surgery programme in the Netherlands: a qualitative study investigating facilitators and barriers for implementation. BMJ Open 2022;12:e051513. [Crossref] [PubMed]
- Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol 2016;33:160-71. [Crossref] [PubMed]
- Fiorelli S, Leopizzi G, Menna C, et al. Ultrasound-Guided Erector Spinae Plane Block Versus Intercostal Nerve Block for Post-Minithoracotomy Acute Pain Management: A Randomized Controlled Trial. J Cardiothorac Vasc Anesth 2020;34:2421-9. [Crossref] [PubMed]
- Borys M, Żurek S, Kurowicki A, et al. Implementation of Enhanced Recovery After Surgery (ERAS) protocol in off-pump coronary artery bypass graft surgery. A prospective cohort feasibility study. Anaesthesiol Intensive Ther 2020;52:10-4. [Crossref] [PubMed]
- Jiao B, Chen H, Chen M, et al. Opioid-sparing effects of ultrasound-guided erector spinae plane block for adult patients undergoing surgery: A systematic review and meta-analysis. Pain Pract 2022;22:391-404. [Crossref] [PubMed]
- Aksu C, Gurkan Y. Defining the Indications and Levels of Erector Spinae Plane Block in Pediatric Patients: A Retrospective Study of Our Current Experience. Cureus 2019;11:e5348. [Crossref] [PubMed]
- Çiftçi B, Ekinci M. Ultrasound-guided single shot preemptive erector spinae plane block for thoracic surgery in a pediatric patient. Agri 2020;32:58-9. [PubMed]
- Tsui BCH, Esfahanian M, Lin C, et al. Moving toward patients being pain- and spasm-free after pediatric scoliosis surgery by using bilateral surgically-placed erector spinae plane catheters. Can J Anaesth 2020;67:621-2. [Crossref] [PubMed]
- Bliss DP Jr, Strandness TB, Derderian SC, et al. Ultrasound-guided erector spinae plane block versus thoracic epidural analgesia: Postoperative pain management after Nuss repair for pectus excavatum. J Pediatr Surg 2022;57:207-12. [Crossref] [PubMed]
- Ivanusic J, Konishi Y, Barrington MJ. A Cadaveric Study Investigating the Mechanism of Action of Erector Spinae Blockade. Reg Anesth Pain Med 2018;43:567-71. [Crossref] [PubMed]
- Schoenfeldt J, Guffey R, Fingerman M. Cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Schwartzmann A, Peng P, Maciel MA, et al. A magnetic resonance imaging study of local anesthetic spread in patients receiving an erector spinae plane block. Can J Anaesth 2020;67:942-8. [Crossref] [PubMed]
- Govender S, Mohr D, Bosenberg A, et al. A cadaveric study of the erector spinae plane block in a neonatal sample. Reg Anesth Pain Med 2020;45:386-8. [Crossref] [PubMed]
- Abduallah MA, Al-Ahwal LA, Ahmed SA. Effect of erector spinae plane block on postoperative analgesia after pediatric hip surgery: Randomized controlled study. Pain Pract 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Moore RP, Liu CJ, George P, et al. Early experiences with the use of continuous erector spinae plane blockade for the provision of perioperative analgesia for pediatric liver transplant recipients. Reg Anesth Pain Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Kaushal B, Chauhan S, Magoon R, et al. Efficacy of Bilateral Erector Spinae Plane Block in Management of Acute Postoperative Surgical Pain After Pediatric Cardiac Surgeries Through a Midline Sternotomy. J Cardiothorac Vasc Anesth 2020;34:981-6. [Crossref] [PubMed]



