
A prediction study of IL-18 and IFN-γ in glucocorticoid treatment response in infants and young children with severe Mycoplasma pneumoniae pneumonia
Introduction
The incidence of Mycoplasma pneumoniae pneumonia (MPP) in infants and young children is increasing in clinical settings. However, MPP in infants and young children is difficult to treat because of the severity of clinical symptoms and the poor response to macrolide antibiotics (1,2).
According to the MPP diagnosis and treatment guidelines, glucocorticoids are recommended for refractory and severe MPP (SMPP) and have achieved good results in adults, adolescents, and school-age children.
However, in infants and young children, the adverse reactions of glucocorticoids cannot be ignored, so whether glucocorticoids are used or not in infants and young children is controversial. In our clinical work and previous research, we are more based on the clinical manifestations, such as fever, cough, chest imaging, etc., as the basis for the use of glucocorticoids, but the effect is not very satisfactory. So, we turned to biomarkers for early specificity indicators. The indicators for early treatment with glucocorticoids, methodology of use, and the evaluation of side effects are all needed to explore (3,4).
We used a retrospective design to analyze the use and effectiveness of glucocorticoids in the treatment of infants and young children with SMPP. Since there are various immune responses to inflammatory factors participating in the pathogenesis of MPP and glucocorticoid treatment, we selected children in our cohort who had an effective glucocorticoid response and detected the inflammatory factors in their initial blood samples after admission. We aimed to identify early specific indicators as a reference for the initiation of glucocorticoid treatment and to guide the clinical use of glucocorticoids. We present the following article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-139/rc).
Methods
Subjects
We enrolled 59 infants and young children with SMPP (aged 1–36 months, mean age 1.99 years ±0.987 months; 35 boys and 24 girls) admitted to Department of Pediatrics, the Sixth People’s Hospital Affiliated to Shanghai Jiaotong University, from January to December 2017. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Jiaotong University (No. 2018-106), and informed consent was obtained from the patients’ parents or legal guardians. Limited by objective conditions, it was difficult to obtain a large number of subjects that meet criteria for age and diagnosis in a short period of time, and we planned to expand the sample size in further study.
Diagnosis of M. pneumoniae infection
M. pneumoniae infection was diagnosed according to the following criteria (5): detection by a particle agglutination test, where the single MP-IgM antibody titer was ≥1:160, or the MP-IgM antibody titer in the recovery and acute phases was four or more times higher than initial results, or re-examination changed from negative to positive as determined by a microparticle agglutination assay (MAG) (Serodia-Myco II, Fujirebio, Tokyo, Japan) (1,6).
Diagnosis of severe pneumonia
A diagnosis of pneumonia was based on both clinical and radiological findings. The severity of pneumonia was assessed on scores ranging from 0 to 5 according to the following number of clinical findings observed during the patients' admission (7,8): fever (>38.5 ℃), rapid breathing (and/or lower chest wall indrawing), decreased oxygen saturation when breathing room temperature air (<92%), more than 7 days of hospital admission, and more than two affected pulmonary lobes identified by chest X-ray. All 59 patients were defined as having severe pneumonia with a severity score ≥3.
The exclusion criteria were as follows: (I) wheezing caused by congenital larynx-tracheal achondroplasia, tracheal foreign body, acute laryngitis, etc.; (II) Mycoplasma pneumoniae associated with other infections; (III) patients diagnosed with bronchial asthma; (IV) long-term or intravenous glucocorticoid use during admission; (V) immunoglobulin and other immunomodulator use during hospitalization or the previous three months.
Clinical data analysis
We collected the medical history and laboratory findings of the admitted infants and young children at the time of admission. The medical history included duration of fever, duration of coughing, and the highest body temperature before admission. The tests included white blood cell count (WBC), C-reactive protein (CRP), platelet (PLT) count, and the initial Mycoplasma pneumoniae (MP) titer on admission. The imaging results included chest X-ray or CT descriptions.
We also collected the clinical outcomes of the enrolled patients after admission. Since not all patients underwent a chest X-ray or a repeat blood test before discharge, we used changes in clinical symptoms and signs to evaluate treatment efficacy. Specific items included length of hospital admission, duration of fever, and duration of coughing and rales while in hospital. Patients were grouped according to whether the treatment was effective or not.
Study grouping and protocol
The normal treatment group consisted of SMPP patients who received standardized treatment with macrolide antibiotics and other supportive treatments (6).
The glucocorticoid treatment group consisted of SMPP patients who had received glucocorticoids intravenously for 3 or more days in addition to normal treatment during their hospitalization. The glucocorticoid used in this study was methylprednisolone (Pfizer Manufacturing Belgium NV).
We compared the clinical characteristics of the normal and glucocorticoid treatment groups in the early days of admission to summarize the patterns for choosing glucocorticoid therapy in clinical practice. We then compared the clinical outcomes of the normal and glucocorticoid groups after treatment to assess whether the empirical treatment plan was effective or not.
The glucocorticoid treatment group was further subdivided into two groups according to treatment efficacy. The effective treatment group comprised patients whose main clinical symptoms and signs significantly improved after treatment (signified by absence of fever, relief of cough, normal mental state and appetite, and absence of complications) (9).
The ineffective treatment group comprised patients with ongoing clinical symptoms and signs, as well as laboratory results that did not significantly improve or that worsened after treatment (9).
As glucocorticoid treatment progressed, children in the ineffective group gradually improved and therefore entered the effective group continuously, thereby forming a dynamic relationship between the effective and ineffective groups and the number of days of glucocorticoid use. The effective and ineffective groups were analyzed separately for each day of glucocorticoid use, from day 1 to day 7.
Potential early specific indicators were compared individually in the effective and ineffective treatment groups over different days of glucocorticoid treatment. These indicators included the duration of fever, maximum body temperature, and duration of coughing at the time of admission. Inflammatory indicators included initial lactate dehydrogenase (LDH) levels, MP titer, IL-18, IL-4, IL-10, IFN-γ, WBC, PLT, erythrocyte sedimentation rate (ESR), and procalcitonin (PCT) at the time of admission. A total of 14 individual indicators were measured to identify specific markers that could prompt the effective early initiation of glucocorticoid treatment.
Evaluation of clinical laboratory data and other cytokines
The following clinical parameters were determined by routine methods: WBC, CRP, PLT, ESR, PCT, MP titer, and LDH.
Other cytokines and chemokines, such as IL-18, IL-4, IL-10, and IFN-γ, were measured with commercially available ELISA kits (BioLegend and PeproTech, USA), and all assays were performed according to each supplier’s recommendations. The specific monoclonal antibody precoated on the microplate was combined with the cytokine in the sample, and then an enzyme-labeled reagent was added for incubation. When the cytokine was present in the sample, a “coated antibody-enzyme-labeled antibody” complex was formed. The HRP on the composite catalyzed the reaction of the developer. The absorbance (A value) was detected by a microplate reader, and the standard curve was fitted with the standard concentration value and the A value to calculate the content of the cytokine in the sample.
Statistical analysis
SPSS 19.0 statistical software (IBM SPSS Statistics 19) was used to analyze the experimental data. Measurement data are expressed as the mean ± standard deviation (x±SD), and independent sample t-tests were used to compare the two groups. Count data are expressed as rate (%) and were analyzed using the chi-square test. A P value <0.05 was two-sided and considered to reflect a statistically significant difference. Logistic regression and factor analysis was used to identify the independent factors associated with glucocorticoid therapy. A receiver operating characteristic (ROC) curve was used to select the optimal cut-off values for the identified risk factors.
Results
Analysis of age and gender composition in SMPP infants
The 59 infants with SMPP were divided into a glucocorticoid treatment group and a normal treatment group. The t-test results for the mean age distribution of the two groups and the chi-square results for the gender distribution composition ratio found no statistical difference between the two groups (P>0.05) (Table 1).
Table 1
| Variables | Glucocorticoid use (N=31) | Normal treatment (N=28) | t/χ2 | P value |
|---|---|---|---|---|
| Age (years) | 1.42±0.927 | 1.03±0.445 | 1.593 | 0.112 |
| Gender, n (%) | ||||
| Male | 18 (58.06) | 15 (53.57) | 0.12 | 0.728 |
| Female | 13 (41.94) | 13 (46.43) |
SMPP, severe Mycoplasma pneumoniae pneumonia.
Analysis of factors associated with glucocorticoid use in SMPP infants
Among the 59 SMPP patients, 31 received glucocorticoid treatment, and 28 received normal treatment. A comparison of the admission information between the two groups revealed statistically significant differences in the number of days of fever before admission and pulmonary imaging findings (P<0.05). However, there was no difference in any other items (Tables 2,3). These findings suggest that current clinical experience identified patients with prolonged fever and severe imaging manifestations as more in need of early empirical glucocorticoid therapy.
Table 2
| Condition on admission | Glucocorticoid use (N=31) | Normal treatment (N=28) | t/χ2 | P value |
|---|---|---|---|---|
| Fever before admission (days) | 7.09±5.70 | 4.39±3.34 | 2.191 | 0.033 |
| Cough before admission (days) | 9.96±7.56 | 7.17±5.54 | 1.16 | 0.251 |
| Maximum temperature before admission | 39.32±0.88 | 39.40±1.29 | −0.26 | 0.796 |
| WBC after admission (109/L) | 10.34±5.42 | 9.67±3.76 | 0.539 | 0.592 |
| CRP after admission (mg/L) | 21.20±19.39 | 16.63±11.17 | 0.795 | 0.43 |
| PLT after admission (109/L) | 355.03±144.39 | 309.85±173.01 | 1.093 | 0.279 |
| MP titer after admission (1:X) | 370.32±258.19 | 253.24±159.14 | 1.784 | 0.081 |
SMPP, severe Mycoplasma pneumoniae pneumonia; MP, Mycoplasma pneumoniae; WBC, white blood cell; CRP, C-reactive protein; PLT, platelet.
Table 3
| Group | n | Inflammatory exudate*# | Segmental opacities | Massive consolidation | χ2 | P value |
|---|---|---|---|---|---|---|
| Glucocorticoid use | 31 | 5 | 18 | 8 | ||
| Normal treatment | 28 | 9 | 17 | 2 | 2.196 | 0.042 |
| Total | 59 | 14 | 35 | 10 |
Compared with the number of segmental opacities, P*=0.319; compared with the number of massive consolidation, P#=0.032. SMPP, severe Mycoplasma pneumoniae pneumonia.
Analysis of the therapeutic effect of glucocorticoid treatment in SMPP infants
After treatment, the therapeutic effects between the two groups were compared. The duration of fever after admission in the glucocorticoid group was significantly shorter than in the normal treatment group (P<0.05), but there were no statistical differences in any other comparisons (Table 4), indicating that treatment is not very satisfactory when based on the current selection criteria for glucocorticoid use.
Table 4
| Effect of glucocorticoid use | Glucocorticoid use (N=31) | Normal treatment (N=28) | t value | P value |
|---|---|---|---|---|
| Days in hospital | 10.16±4.09 | 9.14±3.09 | 1.069 | 0.29 |
| Fever after admission (days) | 3.35±2.19 | 4.52±2.11 | −2.065 | 0.043 |
| Cough after admission (days) | 9.06±3.93 | 7.50±3.95 | 1.521 | 0.134 |
| Duration of rales after admission (days) | 5.13±2.34 | 4.32±2.76 | 1.715 | 0.109 |
SMPP, severe Mycoplasma pneumoniae pneumonia.
Analysis of early specific indicators for glucocorticoid treatment in SMPP
In the 31 cases included in the glucocorticoid treatment group, the relationship between the number of improved patients and the number of days of glucocorticoid use is shown in Table 5.
Table 5
| Duration of glucocorticoid use | SMPP (N=31) |
|---|---|
| 1 day after glucocorticoid use | |
| Effective | 0 |
| Ineffective | 31 |
| 2 days after glucocorticoid use | |
| Effective | 0 |
| Ineffective | 31 |
| 3 days after glucocorticoid use | |
| Effective | 3 |
| Ineffective | 28 |
| 4 days after glucocorticoid use | |
| Effective | 6 |
| Ineffective | 25 |
| 5 days after glucocorticoid use | |
| Effective | 11 |
| Ineffective | 20 |
| 6 days after glucocorticoid use | |
| Effective | 14 |
| Ineffective | 17 |
| 7 days after glucocorticoid use | |
| Effective | 18 |
| Ineffective | 13 |
SMPP, severe Mycoplasma pneumoniae pneumonia.
Group-by-group independent sample t-tests were performed between the effective and ineffective groups over 7 days of glucocorticoid treatment. Early specific indicators included days of fever, maximum body temperature, and days of coughing at admission. Laboratory indicators included a total of 14 single indicators, such as LDH, MP titer, IL-18, IL-4, IL-10, IFN-γ, WBC, PLT, ESR, and PCT at admission. Comparing these early specific indicators between the effective and ineffective glucocorticoid groups, we found no significant differences between the two groups on the 1st, 2nd, 3rd, 4th, and 7th day of glucocorticoid treatment (P>0.05). However, on day 5, the expression levels of IFN-γ (P=0.037) and IL-18 (P=0.034) were found to be significantly different between the two groups, and IFN-γ (P=0.044) continued to show a significant difference on day 6 of glucocorticoid treatment (Table 6).
Table 6
| Days | Group | n | P value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDH (IU/L) | MP titer (1:X) | IL-18 (pg/mL) | IL-4 (pg/mL) | IFN-γ (pg/mL) | IL-10 (pg/mL) | Fever before admission (days) | Cough before admission (days) | Maximum temperature before admission | WBC (109/L) | CRP (mg/L) | PLT (109/L) | ESR (mm/h) | PCT (ng/mL) | |||
| Day 1 | Effective | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ineffective | 31 | |||||||||||||||
| Day 2 | Effective | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ineffective | 31 | |||||||||||||||
| Day 3 | Effective | 3 | 0.123 | 0.448 | 0.152 | 0.493 | 0.579 | 0.166 | 0.512 | 0.842 | 0.131 | 0.396 | 0.335 | 0.947 | 0.394 | 0.643 |
| Ineffective | 28 | |||||||||||||||
| Day 4 | Effective | 6 | 0.658 | 0.696 | 0.062 | 0.121 | 0.324 | 0.214 | 0.531 | 0.461 | 0.199 | 0.071 | 0.940 | 0.291 | 0.118 | 0.530 |
| Ineffective | 25 | |||||||||||||||
| Day 5 | Effective | 11 | 0.593 | 0.603 | 0.034 | 0.142 | 0.037 | 0.108 | 0.559 | 0.228 | 0.337 | 0.065 | 0.528 | 0.280 | 0.718 | 0.551 |
| Ineffective | 20 | |||||||||||||||
| Day 6 | Effective | 14 | 0.922 | 0.0.994 | 0.201 | 0.174 | 0.044 | 0.742 | 0.160 | 0.054 | 0.119 | 0.424 | 0.160 | 0.276 | 0.893 | 0.164 |
| Ineffective | 17 | |||||||||||||||
| Day 7 | Effective | 18 | 0.851 | 0.784 | 0.213 | 0.307 | 0.194 | 0.762 | 0.152 | 0.649 | 0.502 | 0.203 | 0.597 | 0.319 | 0.878 | 0.305 |
| Ineffective | 13 | |||||||||||||||
SMPP, severe Mycoplasma pneumoniae pneumonia.
Since both IL-18 and IFN-γ appeared on the 5th day of glucocorticoid application, Logistic regression analysis was performed. The results suggest that IL-18 was an independent risk factor for SMPP glucocorticoid application, and IFN-γ was a protective factor (Table 7).
Table 7
| Risk factor | Standard error | P value | OR | 95% CI | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Age (years) | 0.047 | 0.402 | 1.045 | 0.752 | 1.103 |
| Male | 0.106 | 0.196 | 0.977 | 0.749 | 1.034 |
| Female | 0.221 | 0.105 | 1.103 | 0.844 | 1.156 |
| LDH | 0.043 | 0.428 | 1.086 | 1.001 | 1.010 |
| MP titer | 0.032 | 0.435 | 0.997 | 0.994 | 1.000 |
| IL-18 | 0.121 | 0.006 | 1.398 | 1.103 | 1.773 |
| IL-4 | 0.298 | 0.585 | 1.117 | 0.655 | 2.117 |
| IFN-γ | 0.096 | 0.011 | 0.783 | 0.650 | 0.946 |
| IL-10 | 0.216 | 0.747 | 0.933 | 0.611 | 1.425 |
| Fever before admission (days) | 0.035 | 0.190 | 0.891 | 0.749 | 1.059 |
| Maximum temperature before admission | 0.236 | 0.846 | 1.047 | 0.659 | 1.662 |
| Cough before admission (days) | 0.089 | 0.592 | 1.018 | 0.951 | 1.092 |
| WBC | 0.051 | 0.200 | 0.936 | 0.847 | 1.036 |
| CRP | 0.015 | 0.426 | 1.012 | 0.983 | 1.041 |
| PLT | 0.002 | 0.061 | 1.005 | 1.000 | 1.009 |
| ESR | 0.018 | 0.490 | 0.988 | 0.954 | 1.023 |
| PCT | 0.530 | 0.406 | 0.280 | 0.014 | 1.062 |
SMPP, severe Mycoplasma pneumoniae pneumonia; MP, Mycoplasma pneumoniae; IL, interleukin; IFN-γ, interferon-γ; LDH, lactate dehydrogenase; WBC, white blood cell; CRP, C-reactive protein; PLT, platelet; ESR, Erythrocyte sedimentation rate; PCT, procalcitonin.
Cut-off value of early specific indicators for glucocorticoid treatment in SMPP
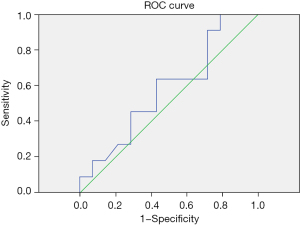
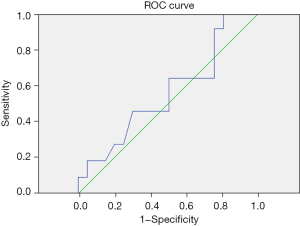
Among the 31 patients treated with glucocorticoids, the newly discovered indicators IL-18 and IFN-γ on the 5th day were measured by an ROC curve to determine their cut-off values for glucocorticoid treatment. IL-18 cut-off value =218.19 pg/mL, AUC (area under the curve) =0.581, sensitivity =0.909, 1-specificity =0.786. IFN-γ cut-off value =11.24 pg/mL, AUC =0.566, sensitivity =0.905, 1-specificity =0.795 (Table 8 and Figures 1,2).
Table 8
| Indicators | ROC curve area | Standard error | P value | 95% CI | Cut-off value | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| IL-18 | 0.581 | 0.117 | 0.494 | 0.352 | 0.910 | 218.19 |
| IFN-γ | 0.566 | 0.135 | 0.549 | 0.301 | 0.831 | 11.24 |
ROC, receiver operating characteristic; SMPP, severe Mycoplasma pneumoniae pneumonia; IL, interleukin; IFN-γ, interferon-γ.
Discussion
MP is one of the most common pathogens causing community-acquired pneumonia in children (3,10). MPP is a type of pneumonia arising from atypical pathogens in children, previously thought to be more common in adolescents and older children. However, in recent years, MP infections have been observed more frequently in younger children, and there are also many infections in infants, most of whom are 9–12 months old (3,9). The principal treatment for MPP is anti-infective treatment, combined with general and symptomatic treatment. Macrolide antibiotics are currently the drug of choice for MPP treatment in children (3,11,12). The general and symptomatic treatment of MPP does not differ from that for CAP in other children (3,9). Macrolide antibiotics bind to the protein of the 23 s special target site of the 50S subunit on the MP ribosome and interfere with the displacement of mRNA by blocking the transpeptidase, thereby selectively inhibiting the synthesis of MP protein.
Inhaled glucocorticoids can be widely used in patients with cough, wheezing, and pulmonary inflammatory lesions identified by chest imaging. The course of treatment ranges from 1 to 3 weeks (13). Systemic glucocorticoid treatment is often used in MPP with acute onset, rapid progression, or severe disease, especially SMPP. A large clinical study confirmed the therapeutic effect of glucocorticoids in SMPP (5). Regular doses are defined as methylprednisolone 1–2 mg/kg/day, and short courses are defined as 3–5 days. Shock therapy can be used when regular doses and courses of treatment are ineffective (13).
Early use of glucocorticoids can inhibit excessive immune responses, reduce the excessive release of inflammatory factors, and protect the body’s tissues and organs. The initiation, dose, and duration of glucocorticoid treatment have been widely discussed among experts around the world, but glucocorticoid usage in special age groups, such as infants and young children, requires further confirmation by large-scale multi-center clinical randomization trials (14,15). Early disease characteristics can sometimes predict the effectiveness of glucocorticoids. Previous studies have found that glucocorticoid therapy may be indicated when prolonged fever exceeds 7 days, the proportion of neutrophils >78%, CRP >110 mg/L, serum ferritin >328 g/L, serum LDH >478 IU/L, and large dense opacities are seen on chest CT (13-15). In our study, we found that LDH and IL-18 levels may be indicators for the initiation of early glucocorticoid treatment in infants and young children with SMPP.
The pathogenesis of pediatric MPP involves various cytokines, and the autoimmune response induced by MP is an important cause of organ damage. The MP-activated NFκb signaling pathway involves the expression of cytokines such as IL-1, IL-6, IL-8, IL-17, IL-18, and TNF-α (16,17). IL-17 and IL-18, as upstream regulators, regulate IL-6 and IL-8 and theoretically can indirectly activate the NFκb signaling pathway (18), so it is speculated that they may be related to RMPP. IL-18 is a member of the IL-1 family and is another cytokine with extensive immunosuppressive activity discovered clinically in recent years. Several studies have shown that IL-18 also plays a crucial role in the autoimmune response of patients with severe pneumonia (18-20). Miyashita et al. found that IL-18 and LDH may be important reference indicators for determining the use of glucocorticoids in RMPP or SMPP, as the serum levels of IL-18 and LDH are positively correlated with the severity of SMPP (6).
There are common antigens found in MP and most organs in the body. When the body is infected with MP, it can produce autoantibodies to form immune complexes, causing cross-immune reactions that induce damage to the lungs and other tissues. However, the immune responses in SMPP tend to be more intense (2,21). LDH is a glycolytic enzyme (22), which exists in the cytoplasm of almost all human body tissues. When tissue cells are injured, LDH will be released to the outside of the cell, causing an increase in this indicator (16). According to Lu et al. (23), total LDH and the expression of four isoenzymes (LDH1, LDH2, LDH4, and LDH5) were significantly higher in an RMPP group than in a non-RMPP group. Logistic regression and factor analysis results also showed that increased levels of ALT, AST, ESR, LDH1, LDH4, and LDH5 were risk factors for RMPP. Kawamata’s study suggested that the level of LDH in plasma can be used as an early indicator for the initiation of glucocorticoid use (24). Narita (25) reported that a plasma LDH level of 302–364 IU/L can be used as a condition for starting glucocorticoid therapy in children with MPP. In treating SMPP and RMPP, the combined use of glucocorticoids with azithromycin or erythromycin can significantly reduce the concentration of LDH in plasma, suggesting that LDH can be used as an indicator for glucocorticoid therapy initiation in SMPP and RMPP. Selected indicators can also be used to measure the effectiveness of treatment.
Acknowledgments
Funding: This work was supported by the Sixth People’s Hospital Affiliated to Shanghai Jiaotong University, Clinical Research Project (ynlc 201809).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-139/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-139/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-139/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Jiaotong University (No. 2018-106) and informed consent was obtained from all patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spuesens EB, Fraaij PL, Visser EG, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 2013;10:e1001444. [Crossref] [PubMed]
- Jacobs E. Mycoplasma pneumoniae: now in the focus of clinicians and epidemiologists. Euro Surveill 2012;17:20084. [Crossref] [PubMed]
- Wang M, Wang Y, Yan Y, et al. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int J Infect Dis 2014;29:18-23. [Crossref] [PubMed]
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515-26. [Crossref] [PubMed]
- Lee KY, Lee HS, Hong JH, et al. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 2006;41:263-8. [Crossref] [PubMed]
- Miyashita N, Kawai Y, Inamura N, et al. Setting a standard for the initiation of steroid therapy in refractory or severe Mycoplasma pneumoniae pneumonia in adolescents and adults. J Infect Chemother 2015;21:153-60. [Crossref] [PubMed]
- Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011;66:ii1-23. [Crossref] [PubMed]
- Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol 2007;56:1625-9. [Crossref] [PubMed]
- Xu YC, Zhu LJ, Xu D, et al. Epidemiological characteristics and meteorological factors of childhood Mycoplasma pneumoniae pneumonia in Hangzhou. World J Pediatr 2011;7:240-4. [Crossref] [PubMed]
- Cho HK. Consideration in treatment decisions for refractory Mycoplasma pneumoniae pneumonia. Clin Exp Pediatr 2021;64:459-67. [Crossref] [PubMed]
- Weare-Regales N, Hudey SN, Lockey RF. Practical Guidance for Prevention and Management of Glucocorticoid-Induced Osteoporosis for the Allergist/Immunologist. J Allergy Clin Immunol Pract 2021;9:1841-50. [Crossref] [PubMed]
- Tsiodras S, Kelesidis I, Kelesidis T, et al. Central nervous system manifestations of Mycoplasma pneumoniae infections. J Infect 2005;51:343-54. [Crossref] [PubMed]
- Tamura A, Matsubara K, Tanaka T, et al. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect 2008;57:223-8. [Crossref] [PubMed]
- de Benedictis FM, Bush A. Corticosteroids in respiratory diseases in children. Am J Respir Crit Care Med 2012;185:12-23. [Crossref] [PubMed]
- Ding S, Wang X, Chen W, et al. Decreased Interleukin-10 Responses in Children with Severe Mycoplasma pneumoniae Pneumonia. PLoS One 2016;11:e0146397. [Crossref] [PubMed]
- Salvatore CM, Fonseca-Aten M, Katz-Gaynor K, et al. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect Immun 2007;75:236-42. [Crossref] [PubMed]
- Lauw FN, Branger J, Florquin S, et al. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol 2002;168:372-8. [Crossref] [PubMed]
- Kurai D, Nakagaki K, Wada H, et al. Mycoplasma pneumoniae extract induces an IL-17-associated inflammatory reaction in murine lung: implication for mycoplasmal pneumonia. Inflammation 2013;36:285-93. [Crossref] [PubMed]
- Schultz MJ, Knapp S, Florquin S, et al. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun 2003;71:1630-4. [Crossref] [PubMed]
- Narita M, Tanaka H. Late increase of interleukin-18 levels in blood during Mycoplasma pneumoniae pneumonia. Cytokine 2012;59:18-9. [Crossref] [PubMed]
- Lee YC, Chang CH, Lee WJ, et al. Altered chemokine profile in Refractory Mycoplasma pneumoniae pneumonia infected children. J Microbiol Immunol Infect 2021;54:673-9. [Crossref] [PubMed]
- Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep 2017;7:45194. [Crossref] [PubMed]
- Lu A, Wang C, Zhang X, et al. Lactate Dehydrogenase as a Biomarker for Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children. Respir Care 2015;60:1469-75. [Crossref] [PubMed]
- Kawamata R, Yokoyama K, Sato M, et al. Utility of serum ferritin and lactate dehydrogenase as surrogate markers for steroid therapy for Mycoplasma pneumoniae pneumonia. J Infect Chemother 2015;21:783-9. [Crossref] [PubMed]
- Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 2010;16:162-9. [Crossref] [PubMed]


