
Clinical features and prognosis of acute lymphoblastic leukemia in children with Epstein-Barr virus infection
Introduction
Acute lymphoblastic leukemia (ALL) is a relatively common clinical malignant disease of the hematopoietic system with an abnormal clone of bone marrow lymphoid group cells. The abnormal proliferation of diseased lymphocytes is not functional, and the infiltration of malignant cells inhibits the normal hematopoietic function of the bone marrow, which in turn produces the corresponding symptoms (1-4). Although ALL can occur at any age, it is more prevalent in children and adolescents, and has become an important issue affecting the health of our nation. Clinical studies have shown (5-8) that ALL is a highly heterogeneous disease with diverse clinical and biological features and a variable prognosis for children. Therefore, identifying the causative factors and prognostic risk factors for ALL has become an urgent challenge. However, until now, the pathogenesis of ALL has remained unclear and it is commonly believed that ALL may be a lesion associated with several factors such as the environment, opportunistic infections, and genetics. Epstein-Barr virus (EBV) is a human herpesvirus type 4 and is commonly found in all age groups, including children and adolescents. Infection with EBV occurs in approximately 90% of the population, but people with EBV infection usually do not show significant clinical symptoms. Nevertheless, a large body of research evidence (9,10) shows that EBV infection can involve organs throughout the body and is associated with a variety of diseases, such as diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and leukemia. Such involvements are due to the ability of EBV to persist in a quiescent state in memory B cells, leading to latent infection, which can cause abnormal immune function through a variety of mechanisms, thus contributing to the development of a variety of malignancies. It has been reported that EBV infection is associated with a high incidence of childhood leukemia, poorer overall survival rate and shorter survival (11). In recent years, the relationship between EBV infection and the development of leukemia has gradually become the focus of research in China and worldwide, but there is little research on the association between EBV infection and childhood ALL. In this study, the impact of EBV infection on the clinical features and prognosis of childhood ALL was analyzed to clarify its relationship, to provide new ideas for the prognostic assessment of children with ALL. We present the following article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-146/rc).
Methods
Participants
A retrospective consecutive selection of 162 children with ALL admitted to Heilongjiang Provincial Hospital from January 2018 to December 2020. EBV testing was performed on all children after enrollment, and the children were divided into the 2 groups of infected and non-infected according to whether they were EBV infected. The 2 groups were comparable in terms of general information (P>0.05).
The ALL diagnostic criteria were as follows: diagnosis confirmed by bone marrow smear, histochemical staining, clinical features, and in accordance with the World Health Organization (WHO) 2016 relevant diagnostic criteria (12).
Criteria for EBV infection included positivity for viral DNA, capsid antibodies immunoglobulin M (IgM).
The inclusion criteria were as follows: initial diagnosis of ALL, meeting diagnostic criteria, children in the infected group meeting criteria for EBV infection, voluntary participation, informed family consent.
The exclusion criteria were as follows: myelodysplastic syndrome transformed leukemia, mixed phenotype leukemia, immunodeficiency disorders, those who relinquished treatment despite a clear clinical diagnosis of ALL.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Heilongjiang Provincial Hospital (No. 2021-01419) and informed consent was taken from all the patients’ guardians.
Clinical characteristics
General data of children in the 2 groups were collected, including gender ratio, age stratification, first symptoms (fever, anemia, subcutaneous hemorrhage, hepatosplenic lymph node enlargement), karyotype, immunophenotyping, fusion genes, clinical risk, whether secondary infection occurred during chemotherapy [no, yes (lung infection, intracranial infection, intestinal infection, sepsis, sepsis combined with pneumonia)], level of routine blood indicators [white blood cell count (WBC), hemoglobin (Hb), platelet count (PLT)], level of lymphocyte subpopulation indicators (CD3+, CD4+, CD8+, CD4+/CD8+).
Karyotype analysis: bone marrow specimens were collected and determined by the 24-hour culture G banding method, and karyotypes were analyzed according to the International System for Human Cytogenetic Nomenclature (ISCN 2009) (13), including normal karyotypes and abnormal karyotypes (undivided, polyploid, subdiploid, abnormal structure).
Immunophenotyping: bone marrow specimens were taken and immunophenotypes, including T-cell and B-cell types, were tested using a flow cytometer [BD FACSCalibur; Becton, Dickinson, and Co., (BD), Franklin Lakes, NJ, USA].
Fusion genes: bone marrow specimens were taken and the fusion genes, including TEL/AML, BCR/ABL, and MLL, were determined using real-time fluorescent quantitative polymerase chain reaction (fluorescent RT-qPCR).
Clinical risk level: The Chinese Children’s Cancer Group ALL-2015 (CCCG-ALL-2015) protocol was used to assess risk level. The risk classification criteria were low-, intermediate-, and high-risk. Low-risk was classified as (I) 1 year ≤age <10 years and WBC ≤50×109/L, chromosome count ≥50 or DNA index ≥1.16, TEL/AML fusion genotype; (II) CNS3 or testicular leukemia, t(1;19), t(9;22), MLLr, chromosome count <44, 19d microscopic residual disease ≥1%. Intermediate risk was classified as: (I) Ph+ALL; (II) T-ALL; (III) MLLr: age ≥1 year or WBC <300×109/L, chromosome count <44. High-risk was classified as: (I) 46 days microscopic residual disease ≥1%; (II) MLLr: age <1 year or WBC ≥300×109/L.
Routine blood count, lymphocyte subset indicators: Routine blood parameters were collected including WBC, Hb, PLT, and lymphocyte subset indicators (CD3+, CD4+, CD8+, CD4+/CD8+) levels at the time of initial diagnosis.
Prognosis assessment
All children were stratified according to their risk level and treated with early intensive chemotherapy, late weak chemotherapy, and staged and long-term standardized treatment. All children were followed up for 12 months and the prognosis, including complete remission rate at 46 days after chemotherapy, complete remission recurrence rate, overall survival (OS), event-free survival (EFS), and survival rate, was calculated. The OS was defined as time from initial diagnosis to final follow-up or death; EFS as time from initial diagnosis to an event (hepatosplenomegaly or infection) or final follow-up; and survival rate as the survival from the initial diagnosis to the last follow-up.
Statistical analysis
Data were analyzed using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA). Kolmogorov-Smirnov was used to test whether the data conformed to a normal distribution. Normally distributed measurement data were expressed as () and independent samples t-tests were used for comparison between 2 groups. Measurements that did not conform to a normal distribution and comparisons between the 2 groups were made using the Mann-Whitney test. Statistical data were expressed as n (%), the χ2 test was applied for comparison between groups, and the Ridit test for rank data. Logistic regression analysis of factors associated with EBV infection with an odds ratio (OR) >1 suggested that the factor was associated with EBV infection. Survival curve analysis was performed using the Kaplan-Meier method; risk ratio (HR) and 95% confidence interval were performed using Cox regression. Receiver operating characteristic (ROC) curves were used to analyze the predictive value of EBV infection on the prognosis of children with ALL. A P value <0.05 indicated statistical significance. All statistics were tested using a 2-sided test with a test level of α=0.05.
Results
Comparison of clinical features between the 2 groups
There was no statistical significance in the comparison between the 2 groups in terms of gender ratio, age distribution, fusion genes, and characteristics of routine blood indicators WBC, Hb, and PLT (P>0.05). There was statistical significance in the comparison between the 2 groups in terms of characteristics of first symptoms, karyotype, immunophenotyping, clinical risk, whether secondary infection occurred during chemotherapy, and lymphocyte subpopulation (P<0.05). The clinical features of ALL children infected with EBV were hepatosplenic lymph node enlargement, abnormal karyotype, B-cell phenotype, high risk of disease, secondary infection during chemotherapy, reduced CD3+, CD4+, CD4+/CD8+, and elevated CD8+ (Table 1).
Table 1
| Variable | Category | EBV infected group (n=101) | EBV non-infected group (n=61) | χ2/t/P value |
|---|---|---|---|---|
| Gender | Male | 51 (50.50) | 26 (42.62) | 0.945/0.331 |
| Female | 50 (49.50) | 35 (57.38) | ||
| Age (years) | <1 | 4 (3.96) | 2 (3.28) | 0.161/0.923 |
| 1–10 | 32 (31.68) | 21 (34.43) | ||
| >10 | 65 (64.36) | 38 (62.30) | ||
| First symptoms | Fever | 27 (26.73) | 19 (31.15) | 34.650/<0.001 |
| Anemia | 14 (13.86) | 19 (31.15) | ||
| Subcutaneous hemorrhage | 6 (5.94) | 14 (22.95) | ||
| Hepatosplenic lymph node enlargement | 54 (53.47) | 6 (9.84) | ||
| Karyotype | Normal | 36 (35.64) | 42 (68.85) | 16.800/<0.001 |
| Abnormal | 65 (64.36) | 19 (31.15) | ||
| Immunotyping | T-cell type | 13 (12.87) | 55 (90.16) | 93.284/<0.001 |
| B-cell type | 88 (87.13) | 6 (9.84) | ||
| Fusion of genes | Negative | 78 (77.23) | 42 (68.85) | 1.389/0.239 |
| Positive | 23 (22.77) | 19 (31.15) | ||
| Clinical risk level | Low risk | 18 (17.82) | 26 (42.62) | 11.960/0.003 |
| Medium risk | 30 (29.70) | 14 (22.95) | ||
| High risk | 53 (52.48) | 21 (34.43) | ||
| Secondary infections during chemotherapy | Yes | 62 (61.39) | 26 (42.62) | 5.396/0.020 |
| No | 39 (38.61) | 35 (57.38) | ||
| Blood count indicators | WBC (×109/L) | 121.76±12.86 | 122.86±12.64 | 0.531/0.696 |
| Hb (g/L) | 117.86±11.98 | 116.99±11.86 | 0.450/0.654 | |
| PLT (×109/L) | 134.76±13.29 | 135.23±13.76 | 0.215/0.830 | |
| Lymphocyte subsets | CD3+ (%) | 42.13±4.25 | 60.22±6.86 | 20.740/<0.001 |
| CD4+ (%) | 31.87±3.54 | 40.97±5.52 | 12.790/<0.001 | |
| CD8+ (%) | 36.76±4.38 | 30.22±2.87 | 10.390/<0.001 | |
| CD4+/CD8+ | 0.80±0.11 | 0.97±0.13 | 8.892/<0.001 |
EBV, Epstein-Barr virus; WBC, white blood cell count; Hb, hemoglobin; PLT, platelet count; Data means n (%) or mean ± SD.
Analysis of factors associated with EBV infection
The presence or absence of EBV infection in children with ALL was used as the dependent variable (no =0, yes =1) and the meaningful characteristics in Table 1, such as first symptoms, karyotype, immunophenotyping, clinical risk, whether secondary infection occurred during chemotherapy, and lymphocyte subpopulation, were used as independent variables in a logistic regression model. The results suggest that first symptoms, karyotype, immunophenotyping, clinical risk, whether secondary infection occurred during chemotherapy, and lymphocyte subsets are independently associated with EBV infection in children with ALL (P<0.05) (Table 2).
Table 2
| Factors | HR | 95% CI | P value |
|---|---|---|---|
| First symptoms | 3.675 | 2.469–4.421 | 0.001 |
| Karyotype | 3.897 | 2.167–5.332 | 0.001 |
| Immunotyping | 2.812 | 1.098–3.876 | 0.014 |
| Clinical risk level | 3.228 | 2.385–5.623 | 0.003 |
| Secondary infections during chemotherapy | 3.140 | 2.187–4.876 | 0.005 |
| Lymphocyte subsets | |||
| CD3+ (%) | 2.165 | 1.256–3.338 | 0.041 |
| CD4+ (%) | 2.187 | 1.324–3.176 | 0.033 |
| CD8+ (%) | 2.365 | 1.564–3.338 | 0.029 |
| CD4+/CD8+ | 2.189 | 1.616–2.876 | 0.032 |
Assignment: first symptom (non-hepatosplenic lymph node enlargement =0, hepatosplenic lymph node enlargement =1), karyotype (normal =0, abnormal =1), immunophenotyping (T-cell type =0, B-cell type =1), clinical risk (low risk =0, intermediate risk =1, high risk =2), whether secondary infection occurred during chemotherapy (no =0, yes =1), entry of original value of lymphocyte subpopulation. EBV, Epstein-Barr virus; HR, hazard ratio; CI, confidence interval.
Comparison of the prognosis of the 2 groups
The complete remission rate at 46 days after chemotherapy, EFS, OS, and survival rate were lower in the infected group than in the non-infected group, and the complete remission recurrence rate was higher than in the non-infected group, which was statistically significant (P<0.05) (Table 3). The survival curves of the 2 groups are shown in Figure 1.
Table 3
| Outcomes | EBV infected group (n=101) | EBV non-infected group (n=61) | χ2/t/P value |
|---|---|---|---|
| Complete remission rate at 46 d after chemotherapy (%) | 50.50 (51/101) | 73.77 (45/61) | 8.534/0.003 |
| Complete remission recurrence rate (%) | 27.45 (14/51) | 6.67 (3/45) | 7.087/0.008 |
| EFS (months) means ± SD | 6.13±1.00 | 8.32±0.97 | 13.660/<0.001 |
| OS (months) means ± SD | 8.85±1.14 | 9.21±1.02 | 2.025/0.045 |
| Survival rate (%) | 76 (75.25) | 55 (90.16) | 5.468/0.019 |
EBV, Epstein-Barr virus; EFS, event-free survival; OS, overall survival.
Analysis of the predictive value of EBV infection on the prognosis of children with ALL
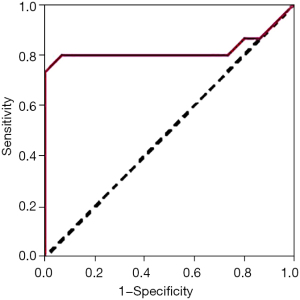
Children in the infected group were divided into a good prognosis group and poor prognosis group according to their survival prognosis. The EBV DNA levels in the good prognosis group were statistically lower than those in the poor prognosis group (P<0.01) (Table 4). The ROC curve showed that EBV had an AUC of 0.921, a sensitivity of 86.57%, and a specificity of 80.16% for predicting prognosis in children with ALL (Figure 2).
Table 4
| Factors | Good prognosis group (n=76) | Poor prognosis group (n=25) | t/P value |
|---|---|---|---|
| EBV DNA (×103 copies/L) | 1.07±0.25 | 8.86±1.14 | 56.130/<0.001 |
EBV, Epstein-Barr virus; ALL, acute lymphoblastic leukemia.
Discussion
The EBV is DNA virus of the γ subfamily, composed of lipid bilayer, double-stranded DNA and capsid. The target cells of this virus are lymphocytes. It exists in a quiescent state in memory B cells with a long latency period. Primary EBV infection is asymptomatic, and this virus can induce malignancy through several pathogenic mechanisms (14-16). Childhood ALL is relatively common in clinical practice, and although many studies have analyzed the mechanisms of childhood ALL, no specific mechanisms have been identified, and it is generally accepted that the disease is associated with several factors, including the environment, opportunistic infections, and genetics (17). In recent years, studies on the relationship between EBV infection and childhood ALL have gradually increased, but fewer studies have been reported on the impact of this viral intervention on the clinical features and prognosis of childhood ALL. In this context, this paper analyzed the clinical characteristics and prognosis of childhood ALL with EBV infection, in order to provide a clinical reference for the study of the relationship between them. The results of this paper showed that 101 out of 162 children with ALL were positive for EBV, a positive rate of 62.35%, indicating a high rate of EBV infection in children with ALL.
In this paper, we compared the clinical features of EBV-infected and non-infected children and found differences in the first symptoms, karyotype, immunophenotyping, clinical risk, secondary infection during chemotherapy, and lymphocyte subsets, suggesting that EBV infection is associated with these clinical features. More than half of primary EBV infections occur in children under 5 years old, such children present only with respiratory infections or infectious mononucleosis, and EBV infection is associated with hepatosplenomegaly in children (18,19). The results of this paper showed that the highest percentage of first symptoms were hepatosplenic lymph node enlargement in infected patients compared to non-EBV infected children, which further supports the conclusion that EBV infection can cause hepatosplenomegaly and is consistent with the characteristics of this virus. It has been shown (20,21) that EBV primarily infects B lymphocytes by viral glycoprotein binding to complement receptors on the surface of B lymphocytes. The results of this paper found that EBV infection was associated with the immunophenotype of the children, with the number of B-cell type cases being higher than the T-cell type in the infected group, which further suggests that the target cells of EBV are B-cells. However, a study has shown (22) that EBV can also infect mononuclear macrophages, natural killer cells, and T lymphocytes. This paper found that 12.87% of EBV-infected children were of the T-cell type and that there was a significant difference in the T-lymphocyte subpopulation index between these children and non-infected children, suggesting that EBV can infect T-lymphocytes in addition to B-lymphocytes, thus affecting the relevant index.
It has been found (23) that the occurrence of ALL is associated with specific karyotypes, with more than 50% of children having abnormal karyotypes. Infection with EBV can prompt chromosomal mutations (24). The results of this paper showed a high proportion of karyotypic abnormalities in childhood ALL with EBV infection and regression analysis showed that karyotypic abnormalities were associated with EBV infection, suggesting that EBV infection is associated with chromosomal abnormalities in ALL. However, no studies have analyzed the relationship between the 2, and the mechanism of association has not been analyzed in this paper, so further studies are needed to verify this.
As the incubation time of EBV increases, the host’s internal environment is disturbed, the immune system is disrupted, and immune impairment occurs, which in turn leads to a reduction in the body’s ability to resist external aggression and an increased risk of infection (25-27). The results of this paper show that there is a significant difference between the 2 groups of childhood ALL in terms of clinical risk and whether infection occurred during chemotherapy, which is related to EBV infection, suggesting that childhood ALL patients with EBV infection have a weakened immune system and attention should be given to the occurrence of infection during treatment. These children are at high risk of disease and should be given priority attention to improve their prognosis and reduce the risk of adverse events.
As the current clinical research on EBV and ALL progresses, it has been found that EBV infection may play an important role in the development and progression of ALL and is an independent predictor of poor prognosis. Tang et al. (28) concluded that EBV infection was independently associated with ALL prognosis. In this study, we analyzed the impact of EBV infection on the prognosis of childhood ALL and showed that childhood ALL with EBV infection had lower rates of complete remission at 46 days, EFS, OS, survival, and higher rates of relapse in complete remission, suggesting that EBV infection can affect the prognosis of childhood ALL and that most childhood ALL have a poor prognosis, consistent with the results of the above study. Further ROC curve analysis of the predictive value of EBV infection on the prognosis of childhood ALL revealed that the AUC was >0.7, indicating that EBV has a high predictive value. The clinical prognosis of EBV-infected childhood ALL can be predicted based on the level of EBV DNA, and timely interventions can be taken to improve survival rates.
Although the results of this paper suggest that EBV infection can affect the clinical features and prognosis of childhood ALL, the specific mechanisms of action have not been analyzed in depth and therefore further analysis is needed in subsequent studies in the hope of benefiting more children with ALL.
In summary, the results of this paper show that children with ALL who are EBV positive are characterized clinically by hepatosplenic lymph node enlargement, low prognostic survival rate, low chemotherapy remission rate, and poor prognosis, and can influence immunophenotyping, clinical risk, and whether secondary infection occurs during chemotherapy, so clinical prediction of childhood ALL and prognosis can be based on EBV infection. Therefore, the condition and prognosis of ALL in children can be predicted according to EBV infection.
Acknowledgments
Funding: This study was supported by Scientific Research Project of Health and Family Planning Commission of Heilongjiang Province (2018220): “Study on IQ, attention, and social function of children with ADHD before and after drug intervention”; Scientific Research Project of Health and Family Planning Commission of Heilongjiang Province (20210606040297): “Correlation between vitamin D and vitamin A and neurobehavioral development of infants”.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-146/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-146/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-146/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Heilongjiang Provincial Hospital (No. 2021-01419) and informed consent was taken from all the patients’ guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coccaro N, Anelli L, Zagaria A, et al. Next-Generation Sequencing in Acute Lymphoblastic Leukemia. Int J Mol Sci 2019;20:2929. [Crossref] [PubMed]
- Xu H, Yu H, Jin R, et al. Genetic and Epigenetic Targeting Therapy for Pediatric Acute Lymphoblastic Leukemia. Cells 2021;10:3349. [Crossref] [PubMed]
- Al-Mahayri ZN, AlAhmad MM, Ali BR. Long-Term Effects of Pediatric Acute Lymphoblastic Leukemia Chemotherapy: Can Recent Findings Inform Old Strategies? Front Oncol 2021;11:710163. [Crossref] [PubMed]
- Douvas MG, Riegler LL. Meeting Challenges in the Long-Term Care of Children, Adolescents, and Young Adults with Acute Lymphoblastic Leukemia. Curr Hematol Malig Rep 2022;17:15-24. [Crossref] [PubMed]
- Kızılocak H, Okcu F. Late Effects of Therapy in Childhood Acute Lymphoblastic Leukemia Survivors Turk J Haematol 2019;36:1-11. [PubMed]
- Xu H, Zhao X, Bhojwani D, et al. ARID5B Influences Antimetabolite Drug Sensitivity and Prognosis of Acute Lymphoblastic Leukemia. Clin Cancer Res 2020;26:256-64. [Crossref] [PubMed]
- Agarwal M, Seth R, Chatterjee T. Recent Advances in Molecular Diagnosis and Prognosis of Childhood B Cell Lineage Acute Lymphoblastic Leukemia (B-ALL). Indian J Hematol Blood Transfus 2021;37:10-20. [Crossref] [PubMed]
- Lee SHR, Antillon-Klussmann F, Pei D, et al. Association of Genetic Ancestry With the Molecular Subtypes and Prognosis of Childhood Acute Lymphoblastic Leukemia. JAMA Oncol 2022;8:354-63. [Crossref] [PubMed]
- Boulanger C, de Ville de Goyet M, de Magnée C, et al. Multiple Epstein-Barr Virus-associated Smooth Muscle Sarcomas of the Gut in a Child Treated for Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol 2019;41:145-7. [Crossref] [PubMed]
- Raastad K, Huang Q. Burkitt leukemia with immature B-cell phenotype mimicking acute lymphoblastic leukemia. Blood 2021;137:718. [Crossref] [PubMed]
- Loutfy SA, Abo-Shadi MA, Fawzy M, et al. Epstein-Barr virus and cytomegalovirus infections and their clinical relevance in Egyptian leukemic pediatric patients. Virol J 2017;14:46. [Crossref] [PubMed]
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391-405. [Crossref] [PubMed]
- Shaffer LG, Slovak ML, Campbell LJ. ISCN 2009: an international system for human cytogenetic nomenclature (2009): recommendations of the International Standing Committee on human cytogenetic nomenclature. Basel: Karger, 2009:6-128.
- Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol 2019;72:651-8. [Crossref] [PubMed]
- Cao Y, Xie L, Shi F, et al. Targeting the signaling in Epstein-Barr virus-associated diseases: mechanism, regulation, and clinical study. Signal Transduct Target Ther 2021;6:15. [Crossref] [PubMed]
- Peng X, Zhou Y, Tao Y, et al. Nasopharyngeal Carcinoma: The Role of the EGFR in Epstein-Barr Virus Infection. Pathogens 2021;10:1113. [Crossref] [PubMed]
- Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica 2020;105:2524-39. [Crossref] [PubMed]
- Cheng H, Chen D, Peng X, et al. Clinical characteristics of Epstein-Barr virus infection in the pediatric nervous system. BMC Infect Dis 2020;20:886. [Crossref] [PubMed]
- Amir R, Kichloo A, Singh J, et al. Epstein-Barr Virus Versus Novel Coronavirus-Induced Hemophagocytic Lymphohistocytosis: The Uncharted Waters. J Investig Med High Impact Case Rep 2020;8:2324709620950107. [Crossref] [PubMed]
- Wang LW, Shen H, Nobre L, et al. Epstein-Barr-Virus-Induced One-Carbon Metabolism Drives B Cell Transformation. Cell Metab 2019;30:539-555.e11. [Crossref] [PubMed]
- Kim TY, Min GJ, Jeon YW, et al. Impact of Epstein-Barr Virus on Peripheral T-Cell Lymphoma Not Otherwise Specified and Angioimmunoblastic T-Cell Lymphoma. Front Oncol 2022;11:797028. [Crossref] [PubMed]
- Liu J, Yan C, Zhang C, et al. Late-onset Epstein-Barr virus-related disease in acute leukemia patients after haploidentical hematopoietic stem cell transplantation is associated with impaired early recovery of T and B lymphocytes. Clin Transplant 2015;29:904-10. [Crossref] [PubMed]
- Münz C. Co-Stimulatory Molecules during Immune Control of Epstein Barr Virus Infection. Biomolecules 2021;12:38. [Crossref] [PubMed]
- Kim KD, Tanizawa H, De Leo A, et al. Epigenetic specifications of host chromosome docking sites for latent Epstein-Barr virus. Nat Commun 2020;11:877. [Crossref] [PubMed]
- Münz C. Cytotoxicity in Epstein Barr virus specific immune control. Curr Opin Virol 2021;46:1-8. [Crossref] [PubMed]
- Latour S, Fischer A. Signaling pathways involved in the T-cell-mediated immunity against Epstein-Barr virus: Lessons from genetic diseases. Immunol Rev 2019;291:174-89. [Crossref] [PubMed]
- Iizasa H, Kim H, Kartika AV, et al. Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases. Front Immunol 2020;11:367. [Crossref] [PubMed]
- Tang S, Zhang R, Wang D, et al. Correlation of Epstein-Barr Virus Infection with Prognosis of Children with Acute B Lymphoblastic Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019;27:769-76. [PubMed]



