
Recombinant human growth hormone in the treatment of C.836A/G-caused short stature in a girl: a case report and literature review
Introduction
Short stature (also referred to short stature) refers to individuals of the same race, sex and age whose height is less than 2 standard deviations (-2SD) of the average height of the normal population, or less than the 3rd percentile, under similar living environment (1). At present, the incidence of short stature is 2–3%, and the treatment for short stature is not satisfying (2). The major factors causing short stature include variations in genes and chromosomes or a loss of growth space due to environmental, nutritional, disease, endocrine, and other changes (1). Among the above factors, chromosomal abnormalities or single-gene defects are the more common reasons for the occurrence of short stature (3). C.836A/G means that heterozygous missense mutations occur in coding exon 7 of the PTPN11 gene, causing the transformation of amino acid 279 from tyrosine to cysteine (p.Y279C). It has been reported that C.836A/G results in the occurrence of LEOPARD syndrome in 5-year-old children, which affects the growth and development of those children (4).
At present, growth hormone (GH) is the main treatment method for short stature (5). Clinically, recombinant human growth hormone (rhGH) is commonly applied to improve the height of children with short stature. RhGH belongs to a class of exogenous growth hormone, which has the same effect as growth hormone. It has the same effect as growth hormone and is effective in the treatment of diseases caused by insufficient growth hormone secretion (6). RhGH was approved to treat growth hormone deficiency (GHD) in 1985 and idiopathic short stature in 2003 by the Food and Drug Administration (7). After 30 years of application, the indications of rhGH, including those in the treatment of short stature caused by GHD and other factors, have expanded worldwide. RhGH has been shown to effectively improve the height of children with achondroplasia within 5 years (8). Additionally, a previous study has noted that rhGH is effective in the treatment of short stature, and promotes the growth of these children and increases their bone age and growth rate with high safety (9). However, the effect of rhGH in the treatment of patients with short stature caused by genetic mutations is unclear. This study reports the case of a 5-year-old child with C.836A/G-caused short stature who was treated with rhGH. The efficacy and safety of rhGH were evaluated to provide a new direction and data support for the treatment of short stature. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-174/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A female child aged 5 years and 5 months old was treated at our hospital for growth retardation of >5 years. The child, who was delivered at 36 weeks of gestation due to inadvertent wrestling, had a birth weight of 3.6 kg and a birth length of 50 cm. Some 5 years after her birth, the growth rate of the child was slower than that of normal children, and the child had a height of 71.5 cm at 1 year of age, 82.5 cm at 2 years of age, and 104.6 cm at 5.5 years of age. Additionally, the child was a slightly picky eater, had good sleep quality (she often fell asleep after 21:00), and did not exercise much before the age of 3–4 years.
The child had 1 incident of choking on milk at birth in the hospital, and the respiratory tract was treated routinely without other special treatment. During breastfeeding, the child suffered multiple apneas (without cyanotic manifestations), which recovered after the flapping stimulation of the mother, and after 30 days, the similar conditions disappeared. The child first began to be fed complementary foods at 6 months of age and transitioned to general foods after the age of 1 year. Approximately 1 month after birth, a patent foramen ovale was found in the child during hospitalization due to jaundice. Afterwards, the child underwent multiple echocardiographic examinations, and was found to have a local thick interventricular septum and mild tricuspid regurgitation. At the age of 2 years and 7 months, an echocardiographic examination revealed the spontaneous closure of the foramen ovale. During the period of the examination, the child did not complain of discomfort. After birth, the child failed to pass a hearing test in both ears, and was diagnosed with nerve deafness but did not receive any special treatment. The child was able to communicate normally, and her life and learning were not affected.
The mother had café-au-lait spots and was found to have a history of polycystic ovarian syndrome after marriage. The father was healthy. A sister, who was born in March 2009, was healthy. There was no history of genetic diseases, chronic and infectious diseases, such as diabetes, hypertension, hepatitis, or cancer in the 3-generation lineal and collateral families. In the paternal family, none of the members reached the adult average standard for height except the grandmother and the father. In the maternal family, none of the members reached the adult average standard for height.
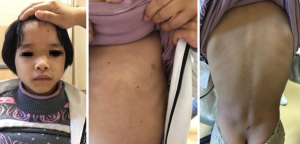
A physical examination was performed on the child, and the results showed normal intelligence, wide eye distance, and a great many freckles with a diameter about 2–5 mm on the skin of the whole body. Further, the child had a height of 104.6 cm (upper/lower body =49.2 cm/55.3 cm =0.89), a weight of 15 kg, a body mass index of 13.71 kg/m2, a head circumference of 48.5 cm, a waist circumference of 48 cm, and a hip circumference of 53 cm. Both breasts were at the B1 stage, and the perineum was at the P1 stage. No obvious deformity was observed in the thorax, and no obvious pathological murmur was observed in cardiac auscultation. The liver and spleen were not significantly enlarged under the ribs (see Figure 1). An auxiliary examination of the child showed normal magnetic resonance imaging results. The peak value of GH in the provocation test of arginine combined with levodopa was 16.93 µg/L and insulin-like factor IGF-1 was 69.19 µg/L, which fell within the normal range. Chromosome was XX, with no abnormality. Trace element content, thyroxine function, liver, and kidney function were normal, and a utero-ovarian B ultrasound showed no abnormality. The child was initially diagnosed with short-stature syndrome, which may be accompanied by gene mutation.
Genetic analysis
Venous blood (2 mL) was collected from the proband and her parents. Next, the genomic deoxyribonucleic acid (DNA) was extracted from the blood using a QIAamp DNA Mini Kit (Qiagen China Co., Ltd., Shanghai, China). Further, whole-exome sequencing was performed on the proband and her parents. Subsequently, according to the results of the whole-exome sequencing and the patient’s direct Sanger sequencing of the exons, C.836A/G (PTPN11) was found in the proband and her parents (see Figure 2).
Clinical efficacy
After careful consideration, the parents of the child agreed to 2.6 units of rhGH (GenSci, Changchun, China) being subcutaneously injected into the child daily. The child’s immune cell level was monitored. Following the injections, no significant changes in lymphocytes and monocytes were observed, and hemoglobin and platelets were also normal in the patient during the application of rhGH for the treatment of C.836A/G. The level of white blood cells and neutrophils declined continuously, but the child did not appear to display clinical symptoms, such as fatigue, dizziness, fever, or bone pain.
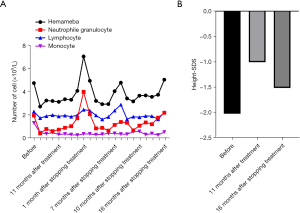
Due to safety concerns, 11 months after the commencement of the treatment, rhGH was discontinued. After discontinuation, take orally Leucogen Tablets to increase leukocytes. After rhGH had been discontinued for 1 month, the level of white blood cells and neutrophils increased abruptly, and then decreased. With prolonged needle withdrawal, the white blood cells and neutrophils gradually returned to normal levels (see Figure 3A). Some 11 months after treatment with rhGH, the height of the patient increased from 106 to 115.6 cm, with a height standard deviation score (SDS) of −1.01. Further, 16 months after stopping treatment, the height SDS of the patient was −1.52, and the height growth rate had slowed down significantly (see Figure 3B).
Discussion
C.836A/G (PTyr279Cys) belongs to the PTPN11 mutation. The PTPN11 gene, located in chromosome 12q24, encodes a protein with the src homology-2 (SH2) domain and a tyrosine phosphatase domain containing the active site. PTPN11 is not only involved in many signal transduction pathways but is also important for normal hematopoietic processes, including proliferation, differentiation, and apoptosis (10). C.836A/G is situated in exon 7 of the PTPN11 gene, and causes amino acid 279 from tyrosine to cysteine (p.Y279C). The above mutation occurs in the SH2 domain of PTPN11. The main function of SH-2 is to mediate the interconnection of multiple signaling proteins in the cytosol to form heteromeric complexes of the proteins, and then to regulate signal transmission in signal transduction pathways. C.836A/G is very rare, and can result in a variety of diseases. C.836A/G-caused diseases, such as LEOPARD syndrome and Noonan syndrome, result in the occurrence of a series of clinical manifestations, including short stature, lentigines, hearing abnormalities, cardiac structural abnormalities, genital dysplasia, and facial anomalies.
A literature search of the PubMed database was conducted using the keywords of “C.836A/G”, “LEOPARD”, “PTPN11”, and “Noonan”, and a literature search of the China National Knowledge Infrastructure and Wanfang database was conducted using the keywords of “C.836A/G”, “LEOPARD syndrome”, “PTPN11”, and “Noonan syndrome”. A total of 17 articles on C.836A/G were retrieved, and 31 cases of C.836A/G were analyzed and collated, of which 26 of the cases were reported abroad (11-23) and 5 were reported in China (24-27). Among the cases, a case reported in Japan was combined with Marfan syndrome (19), a case in Britain (14) and a case in Germany (15) were combined with acute myeloid leukemia, and a case in Bosnia and Herzegovina was combined with growth hormone deficiency (GHD) (18). In all the above reports, the treatment was mainly aimed at the correction of abnormal cardiac structures and abnormal genitalia, the cosmetic treatment of lentigines, and chemotherapy for leukemia. The application of rhGH in the treatment of children with C.836A/G -caused short stature was not mentioned in any of the reports.
In this case, rhGH was chosen to improve the height of the child. Specifically, after 11 months of treatment, the height of the child increased by 9.6 cm, the growth rate reached 10.47 cm/year, and the height SDS increased from −2.03 before treatment to −1.01 after treatment. These findings show the significant benefits of rhGH in relation to height. Some 16 months after stopping treatment, the height of the child had increased by 4.7 cm, the growth rate was 3.5 cm/year, and the height SDS had decreased from –1.01 to –1.52. There were significant differences in the growth rate and height SDS between the injections of rhGH and needle withdrawal, which suggests the good efficacy of rhGH as a treatment. The routine blood results of the child were normal before treatment, but the white blood cells and granulocytes fell and became lower than the normal ranges during treatment. However, after the discontinuation of the medication, the granulocytes gradually returned to the normal range. Thus, the use rhGH in treatment of C.836A/G-caused short stature appears to cause some adverse responses. With the prolonged discontinuation of rhGH, the effect of rhGH on the hematologic system was alleviated, indicating that the discontinuation of rhGH could eliminate adverse responses.
There were 2 cases combined with leukemia among the previous reported articles on C.836A/G, which indicate the risk of hematologic malignancies in C.836A/G (14,15). It has also been reported that the application of rhGH has some common side effects (28), including scoliosis, idiopathic intracranial hypertension, and slipped capital femoral epiphysis. Thus, the occurrence of the adverse responses should be observed in the application of rhGH. It has been found that rhGH combined with other drugs in the treatment of children with short stature effectively promotes growth in height and reduces the occurrence of adverse responses (29). The therapeutic methods of short stature caused by genetic mutations should be further studied using different drug combinations in the future. Currently, foreign treatment data (30-33) have shown that there is no clear relationship between rhGH treatment and malignant tumors (new tumors, tumor recurrence, and secondary tumors). However, in this study, the risk of tumorigenesis needed to be evaluated in the treatment of the child with C.836A/G due to the existence of tumor risks in the genic mutation. During the treatment, routine blood tests should be taken as part of routine monitoring so that any abnormalities in the blood system can be found in a timely manner, and the therapeutic regimen adjusted accordingly.
Conclusions
In summary, rhGH was found to be efficacious in the treatment of C.836A/G-caused short stature. C.836A/G mutation carry intrinsic risk of leukemia, so routine blood test results should be closely monitored during treatment. In relation to the use of rhGH to improve short stature caused by gene mutation syndrome, further investigations need to be conducted to determine its safety, especially in the hematological system.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-174/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-174/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, The Society of Pediatrics, Chinese Medical Association. Guidelines for diagnosis and treatment of children with short stature. Chinese Journal of Pediatrics 2008;46:428-30. [PubMed]
- Li H, Yang ZX. Effect of TGFB1 gene polymorphism on therapeutic effect of recombinant human growth hormone in children with idiopathic short stature. Acta Academiae Medicinae Jiangxi 2021;61:56-60.
- Liang HT. Preliminary study on clinical of characteristics and genetics of short stature caused by rare monogenetic mutations. Peking Union Medical College, 2020.
- Chen YS, Zou MR, Song DY, et al. A Case of LEOPARD Syndrome Caused by PTPN11 Gene Mutation. The Chinese Journal of Dermatovenereology 2021;35:904-7.
- Liang Y. Suggestions on the application of recombinant human growth hormone in pediatric clinical practice. Chinese Journal of Pediatrics 2013;6:426-32.
- Liu Y P, Wang X X, et al. Clinical value of rhGH in the treatment of pediatric dwarfism. Contemporary Medicine 2021;27:88-9.
- Cohen P, Rogol AD, Deal CL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab 2008;93:4210-7. [Crossref] [PubMed]
- Miccoli M, Bertelloni S, Massart F. Height Outcome of Recombinant Human Growth Hormone Treatment in Achondroplasia Children: A Meta-Analysis. Horm Res Paediatr 2016;86:27-34. [Crossref] [PubMed]
- Huang LP. Clinical efficacy and safety of recombinant human growth hormone in the treatment of short stature. Chinese Journal of Clinical Rational Drug Use 2021;14:105-7.
- Pandey R, Saxena M, Kapur R. Role of SHP2 in hematopoiesis and leukemogenesis. Curr Opin Hematol 2017;24:307-13. [Crossref] [PubMed]
- Digilio MC, Pacileo G, Sarkozy A, et al. Familial aggregation of genetically heterogeneous hypertrophic cardiomyopathy: a boy with LEOPARD syndrome due to PTPN11 mutation and his nonsyndromic father lacking PTPN11 mutations. Birth Defects Res A Clin Mol Teratol 2004;70:95-8. [Crossref] [PubMed]
- Martínez-Quintana E, Rodríguez-González F. LEOPARD Syndrome Caused by Tyr279Cys Mutation in the PTPN11 Gene. Mol Syndromol 2012;2:251-3. [Crossref] [PubMed]
- Alfurayh N, Alsaif F, Alballa N, et al. LEOPARD Syndrome with PTPN11 Gene Mutation in Three Family Members Presenting with Different Phenotypes. J Pediatr Genet 2020;9:246-51. [Crossref] [PubMed]
- Uçar C, Calýskan U, Martini S, et al. Acute myelomonocytic leukemia in a boy with LEOPARD syndrome (PTPN11 gene mutation positive). J Pediatr Hematol Oncol 2006;28:123-5. [Crossref] [PubMed]
- Laux D, Kratz C, Sauerbrey A. Common acute lymphoblastic leukemia in a girl with genetically confirmed LEOPARD syndrome. J Pediatr Hematol Oncol 2008;30:602-4. [Crossref] [PubMed]
- Nemes E, Farkas K, Kocsis-Deák B, et al. Phenotypical diversity of patients with LEOPARD syndrome carrying the worldwide recurrent p.Tyr279Cys PTPN11 mutation. Arch Dermatol Res 2015;307:891-5. [Crossref] [PubMed]
- Kim J, Kim MR, Kim HJ, et al. LEOPARD Syndrome with PTPN11 Gene Mutation Showing Six Cardinal Symptoms of LEOPARD. Ann Dermatol 2011;23:232-5. [Crossref] [PubMed]
- Begić F, Tahirović H, Kardašević M, et al. Leopard syndrome: a report of five cases from one family in two generations. Eur J Pediatr 2014;173:819-22. [Crossref] [PubMed]
- Tang S, Hoshida H, Kamisago M, et al. Phenotype-genotype correlation in a patient with co-occurrence of Marfan and LEOPARD syndromes. Am J Med Genet A 2009;149A:2216-9. [Crossref] [PubMed]
- Yoshida R, Nagai T, Hasegawa T, et al. Two novel and one recurrent PTPN11 mutations in LEOPARD syndrome. Am J Med Genet A 2004;130A:432-4. [Crossref] [PubMed]
- Froster UG, Glander HJ, Heinritz W. Molecular genetic mutation analysis of the PTPN11 gene in the multiple lentigines (LEOPARD) syndrome. Hautarzt 2003;54:1190-2. [Crossref] [PubMed]
- Paradisi M, Pedicelli C, Ciasulli A, et al. PTPN11 gene mutation in LEOPARD syndrome. Minerva Pediatr 2005;57:189-93. [PubMed]
- Keren B, Hadchouel A, Saba S, et al. PTPN11 mutations in patients with LEOPARD syndrome: a French multicentric experience. J Med Genet 2004;41:e117. [Crossref] [PubMed]
- Wang Y, Chen C, Wang DW. Leopard syndrome caused by heterozygous missense mutation of Tyr 279 Cys in the PTPN11 gene in a sporadic case of Chinese Han. Int J Cardiol 2014;174:e101-4. [Crossref] [PubMed]
- Zang DJ, Xu XH, Zhou C, et al. Mutation analysis of the PTPN11 gene in a family with LEOPARD syndrome. Chinese Journal of Dermatology 2015;10:429-430.
- Huang SS, Huang BQ, Gao X, et al. Case report and diagnosis of Noonan syndrome with multiple lentigines with deafness as its main clinical feature. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2019;33:804-7. [PubMed]
- Yu SJ, Wang H. A case of LEOPARD syndrome. Chinese Journal of Dermatology. 2018;15:908.
- Watson SE, Rogol AD. Recent updates on recombinant human growth hormone outcomes and adverse events. Curr Opin Endocrinol Diabetes Obes 2013;20:39-43. [Crossref] [PubMed]
- Li YL. Clinical study of rhGH combined with other methods in the treatment of short stature in children. Chinese Journal of Urban and Rural Enterprise Hygiene 2021;36:163-5.
- Kemp SF. Growth hormone treatment of idiopathic short stature: history and demographic data from the NCGS. Growth Horm IGF Res 2005;15 Suppl A:S9-12.
- Cutfield WS, Lindberg A, Rapaport R, et al. Safety of growth hormone treatment in children born small for gestational age: the US trial and KIGS analysis. Horm Res 2006;65:153-9. [PubMed]
- Karavitaki N, Warner JT, Marland A, et al. GH replacement does not increase the risk of recurrence in patients with craniopharyngioma. Clin Endocrinol (Oxf) 2006;64:556-60. [Crossref] [PubMed]
- Bell J, Parker KL, Swinford RD, et al. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab 2010;95:167-77. [Crossref] [PubMed]



