
Effect of family integrated care on physical growth and language development of premature infants: a retrospective study
Introduction
Premature birth (PTB) is defined by the World Health Organization as delivery occurring before 37 weeks of pregnancy or less than 259 days from the 1st day of a woman’s last menstruation (1). According to the gestational weeks of delivery, PTB can be divided into the following categories: (I) very early PTB: PTB occurring at less than 28 weeks of gestation; (II) early PTB: PTB occurring between 28 and 34 weeks of gestation; and (III) light PTB: PTB occurring between 34 and 37 weeks of gestation (2). According to the causes of delivery, PTB can be divided into spontaneous PTB and iatrogenic PTB. Premature delivery not only causes neonatal vision, hearing, respiratory function, and neurological dysfunction (3), but also increases the long-term risk of hypertension, heart disease, and diabetes in adulthood (4). The global disease burden research project in 2010 conducted a systematic study on 291 life-threatening diseases in 21 regions from 1990 to 2010 and proposed that PTB is an independent disease with the largest global burden from the perspectives of high mortality and lifelong health damage (5). Since 1980, the global incidence of PTB has been increasing, and the incidence and economic burden of diseases of the respiratory system, digestive system, circulatory system, and nervous system caused by PTB have also continued to rise. In 2014, the economic loss caused by PTB in Canada was as high as 587 million dollars The economic losses of middle-term premature infants (PIs) and late PIs 10,000 dollars, respectively (6). The global PTB rate was 11.1% in 2010 (7) and 10.6% in 2014 (8). A study of 196 premature and low birth weight infants tracked to the age of 16 found that 37% of children had abnormal brain function and structure, and their executive function and intellectual development were significantly lower than those of normal children (9). A Dutch study followed 1,338 PIs up to the age of 19 and found that the disability rate caused by growth and development defects was increasing. At the age of 5, 24% of children still had delayed language development (10). Therefore, the current management focus of PIs has shifted from ensuring their survival to improving and promoting their growth and development. The main caregivers of PIs after discharge are parents. The nursing ability of parents is closely related to the prognosis and long-term health development outcomes of PIs. At the beginning of 2014, neonatal intensive care unit (NICU) medical staff of Mount Sinai Hospital in Toronto, Canada carried out a study on family integrated care (FIcare), and then conducted a multicenter group randomized controlled study of FIcare in many countries (11-14). The results showed that FIcare can not only improve the prognosis of newborn children, increase the breastfeeding rate, promote weight gain, but also reduce parents’ stress and anxiety, which improves parents’ social well-being. The FIcare clinical study conducted in China in 2014 also confirmed that FIcare can help PIs to reach total enteral feeding faster, lower readmission rate within 30 days after discharge, improve the success rate of breastfeeding, and accelerate the weight gain of PIs (15).
There are few studies on the effects of FIcare on physical development and language development of PIs in China. Our research group has carried out clinical research on the impact of FIcare on the prognosis of PIs in recent years. By inviting the mothers of PIs with stable conditions into the NICU, specialized nurses will train them in skills such as hand hygiene, oral care, skin care, percutaneous oxygen saturation monitoring, bathing, body temperature measurement, diaper changing, kangaroo holding, and breast feeding, essentially making the mothers of PIs become members of the NICU team and participate in the comprehensive management of PIs in hospital. The purpose of this study was to analyze the feasibility of this method and its impact on the prognosis of PIs. Through a retrospective analysis of the growth and development of PIs at the age of 18 months, this study discusses the impact of FIcare on the physical and language development of PIs, to further validate the necessity and importance of FIcare in NICUs in China. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-210/rc).
Methods
Participants
This study retrospectively included 238 PIs (with their mothers) born in the Neonatal Pediatrics Department, Affiliated Hospital of Nantong University from January 2018 to September 2020 and hospitalized in the NICU within 24 hours after birth, as shown in Figure 1. The study was approved by the Ethics Committee of Affiliated Hospital of Nantong University (No. 02020197) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants’ parents provided informed consent.
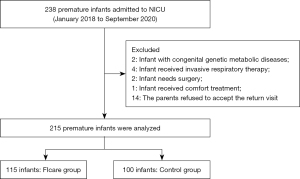
The inclusion criteria were as follows: (I) infants with a gestational age of over 28 weeks and less than 34 weeks; (II) birth weight ≥1,000 and <2,500 g; (III) admitted to the NICU within 24 h after birth; (IV) the parents of the infant had the ability to take care of the infant and possessed basic reading and comprehension skills; and (V) informed consent of parents. The exclusion criteria were as follows: (I) infants with congenital genetic metabolic diseases, digestive tract malformations, severe congenital heart disease, central nervous system and endocrine diseases, or other severe congenital growth and development abnormalities; (II) infant received invasive respiratory therapy; (III) infant required surgery; (IV) infant received comfort treatment; and (V) the parents of the infant had previous mental history.
Intervention
For the control group, the NICU traditional care model was applied for infants, the nurses were responsible for all the nursing of the infants during hospitalization and carried out routine health education to the infant’s parents. The infant’s parents could visit the infants through the ward monitoring system in the hospital every Monday and Friday. At the time of discharge, the responsible nurse carried out discharge education for the baby’s parents, including neonatal nursing methods, precautions, and regular follow-up.
For the FIcare group, a special FIcare group was established, and the ward was transformed accordingly. The mothers of the newborns in the FIcare group were invited to enter the NICU ward. The medical team of the FIcare group guided the parents to learn and complete 13 non-invasive nursing skills, such as the six-step washing technique, adjusting the newborn’s body position, changing diapers and estimating urine volume, umbilical cord care, oral care, kangaroo skin contact, and guided the parents to record the general situation, including body temperature, heart rate, weight, urine, and stool status, milk volume, vitality, and subjective experience, and provide nursing knowledge, skill guidance, and psychological support at any time. At the same time, the mothers were encouraged to communicate with medical staff during daily ward rounds about the baby’s current situation and diagnosis and treatment plan (15). During the FIcare study, parents needed to ensure that they participated in nursing at least 3 hours a day.
Data collection
The researchers collected the basic information about 238 infants and mothers through the medical information system. Through face-to-face communication, telephone follow-up, WeChat, and other means, a return visit was conducted after obtaining informed consent. A total of 215 questionnaire reports were recovered, with an effective recovery rate of 90.34%. This study adopted the method of multivariate analysis, and the sample size needed to be 5–10 times the number of variables. The influencing factors involved in this study, together with the general information of PIs and mothers, included 19 variables. Therefore, the sample size needed for this study could have been between 95 and 190 cases. To increase the reliability of the results, based on the maximum sample size of 190 cases, considering the phenomenon of no response in the survey process and the 20% loss of follow-up rate, 238 questionnaires were planned to be distributed. The actual sample size was 215 cases. The data was collected by fixed researchers and uniformly entered by the designated entry clerk. After the data entry of the last infant was completed according to the estimated sample size, all the data were analyzed by the researchers. The data collected included maternal data, demographic data and hospitalization of PIs, physical development indicators of PIs after discharge, including follow-up of each infant’s weight (weighing before feeding in the morning), head circumference, and body length growth at the age of 1, 3, 6, 12, and 18 months.
Physical growth assessment
To measure weight, the baby was placed in a lying position, their coat and shoes were removed, and the approximate weight of underwear and diapers were subtracted when calculating the weight. The reading was in grams.
To measure body length, the baby’s shoes, hats, and socks were removed. The measurer contacted the top of the child’s head with the top plate of the measuring bed, the ears were at the same level, the trunk was straight, the popliteal fossa contacted the measuring bed, and the pillow, shoulder, hip, popliteal fossa, and heel contacted the measuring board at the same time. The measurer stood on the right side of the child, held the child’s knees with their left hand, and pushed the foot board surface with their right hand to ensure it was in contact with the soles of both feet. The heels were close, and the toes were 60 degrees apart. The foot board surface was at right angles to the bottom plate of the measuring bed, and the readings on both sides were consistent. The reading was in centimeters.
To measure the head circumference, the PI was placed in the supine position, and the measurer used a soft ruler to return to the starting point from the left eyebrow arch, through the temporal bone to the occipital bone, and then to the right eyebrow arch. The reading was in centimeters.
Language development assessment
Neurodevelopmental assessment
The participants were evaluated by Gesell Developmental Schedules. The specific content of the schedules includes five indicators: adaptability, gross motor movement, fine movement, language understanding function, and social contact. The evaluation results are expressed in development quotient (DQ) (16). A DQ >85 is the normal development level, 85–75 is the boundary, and <75 is judged as abnormal.
Language development assessment
The early language milestone (ELM) scale (17) was developed for use in pediatric clinical settings as a brief screening of the language abilities of children under the age of 3 years. It includes auditory expressive ability (26 items), auditory receptive ability (20 items) and visual ability (13 items), with 1 point recorded for each item passed, and 0 points for each item failed. The total score is calculated. Compared with children in the same age group, a score ≤10th percentile (P10) is recorded as abnormal, and a score > P10 is recorded as normal.
Statistical analyses
The statistical software SPSS 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The measurement data were described by mean and standard deviation, and the count data were described by frequency and percentage. The t-test and analysis of variance (ANOVA) were used, and the chi-square (χ2) test was used for counting data. Multivariate logistic regression and multiple linear regression were used to correct for confounding factors. Two-sided test P<0.05 was considered statistically significant.
Results
General information
A total of 238 PIs hospitalized in the NICU were included in this study, of which 215 were followed up effectively and 23 did not receive follow-up. There were 115 PIs in the FIcare group and 100 in the control group. In the FIcare group, there were 50 male PIs and 65 female PIs; the gestational week of delivery was (30.03±1.38) weeks, the birth weight was (1,539.97±333.75) g, the birth length was (39.51±2.07) cm, the birth head circumference was (29.14±1.72) cm, and there were 36 cases of vaginal delivery, and 79 cases of caesarean section. The Apgar score at 5 minutes was (8.56±1.04); the gestational age of the mother was (30.02±4.42) years; there were 39 cases of perinatal complications, and 112 cases of normal birth examination. There were 82 cases of neonatal pneumonia, 92 cases of hyperbilirubinemia, 79 cases of anemia, 27 cases of hypoglycemia, 18 cases of neonatal respiratory distress syndrome (NRDS), 12 cases of bronchopulmonary dysplasia (BPD), 21 cases of non-breastfeeding, 48 cases of breastfeeding, 46 cases of mixed feeding, 21 cases of primary caregivers with less than junior high school education, 46 cases of senior high school and technical secondary school, 48 cases of college or above, 54 cases of family average monthly income <3,000 CNY, 48 cases of 3,000–5,000 CNY, and 13 cases of >5,000 CNY. In the control group, there were 47 male PIs and 53 female PIs; the gestational week of delivery was (29.93±1.30) weeks, birth weight (1,499.25±294.60) g, birth length (39.46±1.91) cm, birth head circumference (28.98±1.47) cm, vaginal delivery occurred in 30 cases, and caesarean section in 79 cases. The Apgar score at 5 minutes was (8.33±1.16); the gestational age of the mother was (29.78±4.21) years; there were 34 cases of perinatal complications, and 96 cases of normal birth examination. There were 67 cases of neonatal pneumonia, 73 cases of hyperbilirubinemia, 71 cases of anemia, 29 cases of hypoglycemia, 8 cases of NRDS, 11 cases of BPD, 18 cases of non-breastfeeding, 55 cases of breastfeeding, 27 cases of mixed feeding, 9 cases of primary caregivers with less than junior high school education, 36 cases of senior high school and technical secondary school, 55 cases of college or above, 41 cases of family average monthly income <3,000 CNY, 38 cases of 3,000–5000 CNY, and 21 cases of >5,000 CNY (Table 1).
Table 1
| Variables | FIcare group (n=115) | Control group (n=100) | t/χ2 | P value |
|---|---|---|---|---|
| Gender (male/female) | 50/65 | 47/53 | 0.268 | 0.605 |
| Gestational age (weeks), | 30.03±1.38 | 29.93±1.30 | 0.57 | 0.569 |
| Birth weight (g), | 1,539.97±333.75 | 1,499.25±294.60 | 0.734 | 0.464 |
| Birth length (cm), | 39.51±2.07 | 39.46±1.91 | 0.194 | 0.846 |
| Birth head circumference (cm), | 29.14±1.72 | 28.98±1.47 | 0.724 | 0.47 |
| Mode of delivery (vaginal delivery/caesarean section) | 36/79 | 30/70 | 0.143 | 0.836 |
| Apgar score at 5 min | 8.56±1.04 | 8.33±1.16 | 1.516 | 0.131 |
| Maternal age (years) | 30.02±4.42 | 29.78±4.21 | 0.402 | 0.688 |
| Perinatal complications (yes/no) | 39/76 | 34/66 | 0.103 | 0.879 |
| Prenatal examination (yes/no) | 112/3 | 96/4 | 0.329 | 0.566 |
| Times of pregnancy (1/2/3/>3) | 34/29/31/21 | 30/24/25/21 | 0.32 | 0.956 |
| Parity (1/2/3) | 71/35/9 | 58/36/6 | 0.882 | 0.643 |
| Neonatal complications | ||||
| Aspiration pneumonia (yes/no) | 82/33 | 67/33 | 0.466 | 0.495 |
| Hyperbilirubinemia (yes/no) | 92/23 | 73/27 | 1.469 | 0.226 |
| Anemia (yes/no) | 79/36 | 71/29 | 1.135 | 0.714 |
| Hypoglycemia (yes/no) | 27/88 | 29/71 | 0.847 | 0.358 |
| NRDS (yes/no) | 18/97 | 8/92 | 2.946 | 0.086 |
| BPD (yes/no) | 12/103 | 11/89 | 0.098 | 0.894 |
| Feeding mode (non-breastfeeding/breast-feeding/mixed feeding) | 21/48/46 | 18/55/27 | 4.628 | 0.099 |
| Primary caregiver education (below junior school/high school and technical secondary school/college degree or above) | 21/46/48 | 9/36/55 | 5.75 | 0.065 |
| Average monthly household income (<3,000/3,000–5,000/>5,000 CNY) | 54/48/13 | 41/38/21 | 3.796 | 0.15 |
NRDS, neonatal respiratory distress syndrome; BPD, bronchopulmonary dysplasia; FIcare, family integrated care.
Comparison of physical growth
There was no significant difference in birth weight, body length, and head circumference between the two groups (P>0.05). The body weight of the FIcare group participants was significantly higher than that of the control group participants at the 1st month of life (P<0.05), but not at the 3rd month (P=0.054). There was no significant difference between the two groups at the 6th, 12th, and 18th months.
The body length and head circumference of FIcare group in the 1st, 3rd, 6th, 12th, and 18th months were significantly higher than those of control group (P<0.05). See Table 2.
Table 2
| Age | FIcare group (n=115) | Control group (n=100) | t | P value |
|---|---|---|---|---|
| Weight (g), | ||||
| Birth | 1,539.97±333.75 | 1,499.25±294.60 | 0.734 | 0.464 |
| 1 month | 3,669.32±3,492.13 | 3,492.13±574.27 | 2.234 | 0.026 |
| 3 months | 5,603.24±1,079.73 | 5,333.32±932.26 | 1.947 | 0.053 |
| 6 months | 7,366.99±931.56 | 7,205.59±893.65 | 1.291 | 0.198 |
| 12 months | 10,072.81±1,035.81 | 9,867.63±1,099.3 | 1.408 | 0.161 |
| 18 months | 11,031.3±997.11 | 10,815.82±1,006.44 | 1.574 | 0.117 |
| Length (cm), | ||||
| Birth | 39.51±2.07 | 39.46±1.91 | 0.194 | 0.846 |
| 1 month | 53.23±5.12 | 52.19±4.86 | 1.527 | 0.128 |
| 3 months | 57.61±5.05 | 55.8±4.58 | 2.735 | 0.007 |
| 6 months | 65.61±3.45 | 64.63±2.68 | 2.299 | 0.022 |
| 12 months | 73.43±3.09 | 72.3±3.1 | 2.659 | 0.008 |
| 18 months | 77.5±2.99 | 78.35±2.91 | 2.095 | 0.037 |
| Head circumference (cm), | ||||
| Birth | 29.14±1.72 | 28.98±1.47 | 0.724 | 0.47 |
| 1 month | 34.37±1.78 | 33.45±1.55 | 3.990 | 0.000 |
| 3 months | 38.33±1.73 | 37.37±1.89 | 3.895 | 0.000 |
| 6 months | 42.49±1.57 | 41.57±1.56 | 4.279 | 0.000 |
| 12 months | 44.78±1.28 | 44.33±1.36 | 2.513 | 0.013 |
| 18 months | 46.59±1.36 | 46.16±1.32 | 2.352 | 0.020 |
FIcare, family integrated care.
Comparison of language development
The DQ score and ELM scale score of FIcare group were significantly higher than those of control group in the 6th, 12th, and 18th months (P<0.05) (Table 3).
Table 3
| Age | FIcare group (n=115) | Control group (n=100) | t/χ2 | P value |
|---|---|---|---|---|
| DQ, | ||||
| 6 months | 88.19±80.63 | 80.63±8.52 | 7.735 | 0.000 |
| 12 months | 90.7±5.49 | 83.47±7.34 | 8.247 | 0.000 |
| 18 months | 90.69±4.31 | 84.33±7.19 | 7.978 | 0.000 |
| ELM scale | ||||
| 6 months (normal/abnormal) | 100/15 | 75/25 | 5.040 | 0.025 |
| 12 months (normal/abnormal) | 110/5 | 85/15 | 7.194 | 0.007 |
| 18 months (normal/abnormal) | 113/2 | 91/9 | 5.809 | 0.016 |
DQ, development quotient; ELM, early language milestone; FIcare, family integrated care.
General factors affecting physical growth of PIs
Taking the weight, length, and head circumference of PIs at 12 months as dependent variables and various variables in the general data questionnaire as independent variables, statistical analysis showed that birth weight, gender, feeding methods, and family per capita monthly income had significant effects on the weight of PIs at 12 months (P<0.05). Intervention methods, gestational age, birth length, gender, and family per capita monthly income had significant effects on the body length of PIs at 12 months (P<0.05). Intervention methods, gestational age, birth head circumference, gender, and family per capita monthly income had significant effects on the head circumference of PIs at 12 months (P<0.05) (Table 4).
Table 4
| Stem | High risk factors | N | Total score, | F | P value |
|---|---|---|---|---|---|
| Weight | Birth weight (g) | 18.285 | 0.000 | ||
| 1,000–1,499 | 86 | 9,393.37±739.69 | |||
| 1,500–1,999 | 114 | 10,239.32±1,056.64 | |||
| 2,000–2,500 | 15 | 11,334.93±697.54 | |||
| Gender | 14.445 | 0.000 | |||
| Male | 97 | 10,273.61±993.14 | |||
| Female | 118 | 9,733.86±1,070.20 | |||
| Feeding mode | 4.839 | 0.009 | |||
| Non breast feeding | 49 | 9,730.38±1,472.05 | |||
| Breast-feeding | 103 | 10,208.46±1,017.60 | |||
| Mixed feeding | 63 | 9,977.38±1,068.24 | |||
| Average monthly household income (CNY) | 9.917 | 0.000 | |||
| <3,000 | 95 | 9,629.60±963.61 | |||
| 3,000–5,000 | 86 | 10,220.93±923.66 | |||
| >5,000 | 34 | 10,333.06±1,387.25 | |||
| Length | Group | 7.071 | 0.008 | ||
| FIcare group | 115 | 73.43±3.09 | |||
| Control group | 100 | 72.30±3.10 | |||
| Gestational age (weeks) | 22.567 | 0.000 | |||
| 28–29 | 85 | 70.33±2.22 | |||
| 30–31 | 101 | 74.05±2.16 | |||
| 32–33 | 29 | 76.45±2.47 | |||
| Birth length (cm) | 18.494 | 0.000 | |||
| 35–38 | 67 | 69.60±2.04 | |||
| 39–41 | 117 | 73.70±1.83 | |||
| 42–44 | 31 | 77.03±1.96 | |||
| Gender | 11.373 | 0.001 | |||
| Male | 97 | 73.68±2.73 | |||
| Female | 118 | 72.26±3.32 | |||
| Average monthly household income (CNY) | 9.667 | 0.000 | |||
| <3,000 | 95 | 71.88±2.99 | |||
| 3,000–5,000 | 86 | 73.71±2.76 | |||
| >5,000 | 34 | 73.70±3.69 | |||
| Head circumference |
Group | 10.957 | 0.013 | ||
| FIcare group | 115 | 44.78±1.28 | |||
| Control group | 100 | 44.33±1.36 | |||
| Gestational age (weeks) | 21.505 | 0.000 | |||
| 28–29 | 85 | 43.43±0.89 | |||
| 30–31 | 101 | 45.15±0.92 | |||
| 32–33 | 29 | 45.90±1.11 | |||
| Birth head circumference (cm) | 18.846 | 0.000 | |||
| 25–28 | 79 | 43.37±1.00 | |||
| 29–31 | 124 | 45.15±0.86 | |||
| 32–34 | 12 | 46.50±1.00 | |||
| Gender | 10.972 | 0.001 | |||
| Male | 97 | 44.90±1.25 | |||
| Female | 118 | 44.31±1.34 | |||
| Average monthly household income (CNY) | 9.829 | 0.000 | |||
| <3,000 | 95 | 44.15±1.25 | |||
| 3,000–5,000 | 86 | 44.93±1.20 | |||
| >5,000 | 34 | 44.88±1.55 |
PI, premature infant; FIcare, family integrated care.
Correlation analysis between physical growth and its influencing factors of PIs
Multiple stepwise regression analysis was carried out with the physical development score of PIs as the dependent variable and the meaningful variable of univariate analysis as the independent variable. The results showed that there was a positive correlation between infant weight and birth weight and family average monthly income at 12 months, and the weight of male infants was higher than that of female infants (P<0.05). Infant length was positively correlated with gestational age, birth length, and family average monthly income. The body length of male infants was longer than that of female infants, and the body length of FIcare group participants was longer than that of control group participants (P<0.05). The head circumference of infants was positively correlated with gestational age, birth head circumference, and family average monthly income. The head circumference of male infants was longer than that of female infants, and the head circumference of FIcare group participants was longer than that of control group participants (P<0.05) (Table 5).
Table 5
| Related factor | B | SE | β | t | P value |
|---|---|---|---|---|---|
| Weight | |||||
| Birth weight (g) | 3.347 | 0.681 | 1.485 | 18.658 | 0.000 |
| Feeding mode | 1.150 | 0.222 | 0.567 | 6.735 | 0.084 |
| Gender | 0.312 | 0.343 | 0.199 | 2.733 | 0.000 |
| Average monthly household income (CNY) | 0.481 | 0.386 | 0.261 | 3.534 | 0.001 |
| Length | |||||
| Group | −0.868 | 0.253 | −0.138 | −3.428 | 0.001 |
| Gestational age (weeks) | 1.526 | 0.206 | 0.331 | 7.411 | 0.000 |
| Birth length (cm) | 3.559 | 0.200 | 0.743 | 17.804 | 0.000 |
| Gender | 0.842 | 0.254 | 0.134 | 3.32 | 0.001 |
| Average monthly household income (CNY) | 0.397 | 0.180 | 0.091 | 2.202 | 0.029 |
| Head circumference | |||||
| Group | −0.412 | 0.118 | −0.154 | −3.484 | 0.001 |
| Gestational age (weeks) | 0.797 | 0.084 | 0.407 | 9.454 | 0.000 |
| Birth head circumference (cm) | 1.58 | 0.105 | 0.678 | 15.079 | 0.000 |
| Gender | 0.284 | 0.120 | 0.106 | 2.377 | 0.018 |
| Average monthly household income (CNY) | 0.400 | 0.082 | 0.217 | 4.89 | 0.000 |
PI, premature infant; SE, standard error.
General factors affecting language development of PIs
Taking the DQ score of PIs as the dependent variable and each variable in the general information questionnaire as the independent variable, the statistical analysis found that the DQ score levels of different intervention methods, gestational age, and general information questionnaire were different, and the difference was statistically significant (P<0.05) (Table 6).
Table 6
| High risk factors | N | Total score, | F | P value |
|---|---|---|---|---|
| Group | 13.221 | 0.000 | ||
| FIcare group | 115 | 90.70±5.49 | ||
| Control group | 100 | 83.47±7.34 | ||
| Gestational age (weeks) | 4.864 | 0.043 | ||
| 28–29 | 85 | 86.12±7.36 | ||
| 30–31 | 101 | 87.69±7.46 | ||
| 32–33 | 29 | 89.69±6.40 |
DQ, development quotient; PI, premature infant; FIcare, family integrated care.
Correlation analysis between language development and its influencing factors in PIs
Multiple regression analysis was carried out with the DQ score of PIs as the dependent variable and a meaningful variable of univariate analysis as the independent variable. The results showed that there was a positive correlation between DQ score and gestational age, and the score of the FIcare group was higher than that of the control group (P<0.05) (Table 7).
Table 7
| Related factor | B | SE | β | t | P value |
|---|---|---|---|---|---|
| Group | −7.107 | 0.872 | −0.483 | −8.152 | 0.000 |
| Gestational age (weeks) | 1.376 | 0.64 | 0.128 | 2.152 | 0.033 |
DQ, development quotient; PI, premature infant; SE, standard error.
Logistic regression analysis was carried out with the ELM scale score of PIs as the dependent variable and the meaningful variable of univariate analysis as the independent variable. The results showed that ELM scale and gestational age were positively correlated with mother’s education, and the score of the FIcare group was higher than that of the control group (P<0.05) (Table 8).
Table 8
| Related factor | B | Ward | P value | OR | 95% CI |
|---|---|---|---|---|---|
| Group | −1.985 | 10.411 | 0.001 | 0.137 | 0.041–0.459 |
| Gestational age (weeks) | −0.298 | 7.082 | 0.009 | 0.742 | 0.495–1.113 |
| Education | 1.629 | 5.405 | 0.020 | 5.101 | 1.291–20.147 |
PI, premature infant; OR, odds ratio; CI, confidence interval.
Discussion
Short term nursing during hospitalization can only help PIs to survive the life-threatening period. In the long term, PIs face ongoing outcome problems such as nervous system dysplasia and language development disorder (18), which seriously affects their individual development and quality of life and brings a heavy burden to the family and society. At present, most NICUs in China still adopt the unaccompanied system. In recent years, some hospitals have successively carried out small-scale kangaroo care (KC) (15) to promote the neural development of newborns, but there is still a lack of standardized clinical research evidence to support allowing parents to enter the NICU and whether this method has an impact on the daily functions of the NICU. The purpose of this study was to encourage the parents of PIs to enter the NICU ward under the guidance of specialists and nurses to participate in the care of their infants, adhere to FIcare, integrate the mother as a member of the infant diagnosis and treatment team, and bring forward the window of time for the mothers of PIs to learn and participate in the care of newborns to the early stage of neonatal hospitalization. This study found that the body length and head circumference of FIcare group participants at the ages of 3, 6, 12, and 18 months were higher than those of the control group participants, indicating that the implementation of FIcare during NICU hospitalization can promote the growth and development of PIs. This promoting effect may be due to FIcare increasing the chance of skin contact between the mother and PIs, making the vital signs of PIs more stable (19). At the same time, the mother’s company helps to slow down the adverse reactions caused by medical operation and environmental stimulation, increase the release of growth hormone, and promote the growth and development of PIs (20). At the same time, the DQ score and language development score of the FIcare group participants were always higher than those of the control group participants from 6 to 18 months of age, which may be related to FIcare improving the breastfeeding rate of PIs and giving PIs more opportunities to have close mother-child communication during hospitalization, which are beneficial to the development of the nervous system (21).
The nervous system of PIs has strong plasticity (22), which is also the theoretical basis of early neurobehavioral intervention in PIs. Neuroplasticity refers to the adaptive changes in the structure and function of the nervous system after stimulation or training. It is manifested in the changes of brain function, behavior, and mental activities in the macro, and in the changes of neural synapses, neurochemicals, neuroelectrophysiology, and so on in the micro. The plasticity mechanism after nerve injury is mainly the reuse of conduction pathways, the formation of new synapses, and the formation of bypass pathways. Compared with the mature brain, the immature brain has the strongest plasticity. Timely and early intervention for PIs can improve the short- and long-term prognoses and reduce neurological disability such as cerebral palsy and intellectual disability. Early intervention makes use of the characteristics of brain development to provide benign environmental stimulation, so that PIs can experience rich positive environmental stimulation in the critical period of brain development after birth, so that the brain can be fully developed and the damaged brain can be repaired to the greatest extent, to help them overcome potential serious problems and ensure that their growth and development reach or catch up with that of normal children. The FIcare model allows parents to enter the NICU to participate in non-medical routine life care during hospitalization of PIs based on the basic principle of mutual respect, competency based training, education, and active participation of parents (23,24). The FIcare model takes parental involvement to a new level, placing families in care centers and making them primary caregivers (25). Not only does FIcare entail the simple participation of the family, but also the change of culture and the relationship between the baby’s parents and hospital staff. In the FIcare model, parents become an integral part of the PI care team, providing active care for infants rather than a passive support role. Parents provide all nursing operations except intravenous fluid management and drug treatment, and the role of nurses is transformed into an educator and guide. After professional education and training, the baby’s family enters the NICU to participate in a range of non-medical nursing of the baby, which can better understand the baby’s growth and various physiological and psychological needs, and cultivate bonding of the mother and baby (26,27). The traditional NICU model implements closed management. The separation of the mother and child undermines the initial bonding between the mother and her child. On the one hand, it has a great psychological impact on the mother, increasing the mother’s negative emotions such as anxiety and depression. On the other hand, breastfeeding is difficult to guarantee. Study in European developed countries have shown that the breastfeeding rate of very low birth weight infants in the NICU is about 50% (28).
The FIcare model circumvents the challenges of mother-infant separation, and family members participate in PI nursing in person. On the one hand, it increases mother-infant contact and interaction, stimulates breast milk secretion, and improves breast-feeding rate; on the other hand, stimulation of the mother’s voice can reduce feeding intolerance in PIs, thereby improving feeding (29). Through mother-infant skin contact, not only does the baby experience a sense of security and satisfaction, but the mother’s sense of confidence and happiness are also increased (30).
Conclusions
Families of PIs play an important role in the nursing and treatment of PIs. Compared with the traditional nursing model, the implementation of FIcare for PIs hospitalized in the NICU has a positive role in promoting the physical growth and language development of infants, and significantly improves the physical development index and language development index of infants at the age of 18 months. Therefore, FIcare represents a new medical model. It is feasible to accompany patients for 3 hours every day, which can be implemented and popularized within the traditional NICU environment. The limitations of this study lie in the relatively small sample size and the data were from a single center, which need to be addressed by conducting further FIcare multi-center clinical research.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-210/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-210/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-210/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Affiliated Hospital of Nantong University (No. 02020197) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants’ parents provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand 1977;56:247-53. [PubMed]
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams obstetrics, 24e. New York: McGraw Hill, 2014.
- Selvanathan T, Guo T, Kwan E, et al. Head circumference, total cerebral volume and neurodevelopment in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2022;107:181-7. [Crossref] [PubMed]
- Duke JW, Lewandowski AJ, Abman SH, et al. Physiological aspects of cardiopulmonary dysanapsis on exercise in adults born preterm. J Physiol 2022;600:463-82. [Crossref] [PubMed]
- Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 2016;21:68-73. [Crossref] [PubMed]
- Johnston KM, Gooch K, Korol E, et al. The economic burden of prematurity in Canada. BMC Pediatr 2014;14:93. [Crossref] [PubMed]
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162-72. [Crossref] [PubMed]
- Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37-46. [Crossref] [PubMed]
- Córcoles-Parada M, Giménez-Mateo R, Serrano-Del-Pueblo V, et al. Born Too Early and Too Small: Higher Order Cognitive Function and Brain at Risk at Ages 8-16. Front Psychol 2019;10:1942. [Crossref] [PubMed]
- van der Pal-de Bruin KM, van der Pal SM, Verloove-Vanhorick SP, et al. Profiling the preterm or VLBW born adolescent; implications of the Dutch POPS cohort follow-up studies. Early Hum Dev 2015;91:97-102. [Crossref] [PubMed]
- O'Brien K, Bracht M, Robson K, et al. Evaluation of the Family Integrated Care model of neonatal intensive care: a cluster randomized controlled trial in Canada and Australia. BMC Pediatr 2015;15:210. [Crossref] [PubMed]
- Ortenstrand A, Westrup B, Broström EB, et al. The Stockholm Neonatal Family Centered Care Study: effects on length of stay and infant morbidity. Pediatrics 2010;125:e278-85. [Crossref] [PubMed]
- O'Brien K, Robson K, Bracht M, et al. Effectiveness of Family Integrated Care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health 2018;2:245-54. [Crossref] [PubMed]
- Murphy M, Shah V, Benzies K. Effectiveness of Alberta Family-Integrated Care on Neonatal Outcomes: A Cluster Randomized Controlled Trial. J Clin Med 2021;10:5871. [Crossref] [PubMed]
- Hei M, Gao X, Gao X, et al. Is family integrated care in neonatal intensive care units feasible and good for preterm infants in China: study protocol for a cluster randomized controlled trial. Trials 2016;17:22. [Crossref] [PubMed]
- Pasamanick B. Gesell and Amatruda's Developmental Diagnosis. Developmental Medicine & Child Neurology 1976;18:397. [Crossref]
- Lewis M. Early Language Milestone Scale. In: Volkmar FR. editor. Encyclopedia of Autism Spectrum Disorders. New York: Springer, 2013:1032-3.
- Romeo DM, Brogna C, Sini F, et al. Early psychomotor development of low-risk preterm infants: Influence of gestational age and gender. Eur J Paediatr Neurol 2016;20:518-23. [Crossref] [PubMed]
- Janvier A, Asaad MA, Reichherzer M, et al. The ethics of family integrated care in the NICU: Improving care for families without causing harm. Semin Perinatol 2022;46:151528. [Crossref] [PubMed]
- Giannì ML, Sannino P, Bezze E, et al. Does parental involvement affect the development of feeding skills in preterm infants? A prospective study. Early Hum Dev 2016;103:123-8. [Crossref] [PubMed]
- Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117:1253-61. [Crossref] [PubMed]
- Parsons E, Claud K, Petrof EO. The infant microbiome and implications for central nervous system development. Prog Mol Biol Transl Sci 2020;171:1-13. [Crossref] [PubMed]
- Jiang S, Warre R, Qiu X, et al. Parents as practitioners in preterm care. Early Hum Dev 2014;90:781-5. [Crossref] [PubMed]
- Lee SK, O'Brien K. Parents as primary caregivers in the neonatal intensive care unit. CMAJ 2014;186:845-7. [Crossref] [PubMed]
- Patel N, Ballantyne A, Bowker G, et al. Family Integrated Care: changing the culture in the neonatal unit. Arch Dis Child 2018;103:415-9. [Crossref] [PubMed]
- Waddington C, van Veenendaal NR, O'Brien K, et al. Family integrated care: Supporting parents as primary caregivers in the neonatal intensive care unit. Pediatr Investig 2021;5:148-54. [Crossref] [PubMed]
- Ou J, Zhong X, Zhang X. Effects of family integrated care on weight gain in extremely preterm infants. Minerva Pediatr (Torino) 2021. [Epub ahead of print]. doi:
10.23736/S2724-5276.21.06070-5 .10.23736/S2724-5276.21.06070-5 - Bonet M, Forcella E, Blondel B, et al. Approaches to supporting lactation and breastfeeding for very preterm infants in the NICU: a qualitative study in three European regions. BMJ Open 2015;5:e006973. [Crossref] [PubMed]
- O'Brien K, Bracht M, Macdonell K, et al. A pilot cohort analytic study of Family Integrated Care in a Canadian neonatal intensive care unit. BMC Pregnancy Childbirth 2013;13:S12. [Crossref] [PubMed]
- Cheng C, Franck LS, Ye XY, et al. Evaluating the effect of Family Integrated Care on maternal stress and anxiety in neonatal intensive care units. J Reprod Infant Psychol 2021;39:166-79. [Crossref] [PubMed]


