
Abnormal electroencephalogram (EEG) after drug withdrawal is a risk factor for epilepsy recurrence in children: a systematic review and meta-analysis
Introduction
Epilepsy is a common neuropsychiatric disease that is characterized by recurrent and transient neurological dysfunction (1,2). Epilepsy mainly occurs in childhood, with patients under the age of 18 years accounting for more than 60% of the total incidence (3). The treatment of epilepsy includes regular, reasonable, and long-term administration of antiepileptic drugs (AEDs); however, taking prolonged medication use will inevitably increase the economic burden. Furthermore, there will also be a series of toxic/side effects that affect the physical and mental health development and treatment of children (4,5). About 90% of children with epilepsy can be fully controlled after early and standardized drug treatment (5). Under complete control of the disease, patients continue to take AEDs for a certain number of years and then cease medication use for observation. However, some children relapse after stopping AEDs, and the recurrence rate is 12–66% (3). Identifying the risk factors of recurrence after drug withdrawal is significant in reducing the recurrence rate. Numerous studies have screened the risk factors of epilepsy recurrence after drug withdrawal (6-10). However, owing to the different research objects and methods involved in these studies, the risk factors identified in each study also differ.
The relationship between electroencephalogram (EEG) abnormalities and epilepsy recurrence after drug withdrawal is controversial. Some studies have pointed out that children with abnormal EEG after drug withdrawal have a higher risk of epilepsy recurrence (11-13). However, a study reached different conclusions. There is no significant difference in the epilepsy recurrence rate between patients with abnormal EEG and normal patients after drug withdrawal and abnormal EEG is not associated with epilepsy recurrence (14). Considering the aforementioned disputes, we conducted a meta-analysis of the literature to clarify the relationship between EEG abnormalities after drug withdrawal and epilepsy recurrence in children. We present the following article in accordance with the MOOSE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-206/rc).
Methods
Literature download
We performed a literature search of the PubMed, EMBASE, Medline, CENTRAL, China National Knowledge Infrastructure (CNKI) and China Science Periodical Database (CSPD) databases for English and Chinese articles on children with epilepsy. The following search terms were used: “children” and “epilepsy” or “epilepsy in children and “drug withdrawal” and “recurrence”.
Literature screening
Inclusion criteria: (I) studies involving subjects who were children with epilepsy; (II) articles involving subjects who stopped taking AEDs; (III) the exposure factors were normal post-withdrawal EEG and abnormal EEG; (IV) studies that observed whether the subjects had epilepsy recurrence; (V) cohort or case-control studies; and (VI) the literature results included the odds ratio (OR) and 95% confidence interval (CI) of epilepsy recurrence in patients with abnormal EEG and normal EEG after drug withdrawal or studies in which this information could be calculated from the data.
Exclusion criteria: (I) repeated reports; (II) studies that included both adults and children as research subjects in which the two could not be distinguished; (III) articles involving subjects who received surgical treatment; and (IV) studies with incomplete literature data that could not be supplemented by contacting the author.
Data extraction
In this paper, two researchers jointly extracted the data from the included literature, including the author, title, publication time, research type, number of researchers, number of epileptic relapses, and number of normal and abnormal EEGs. Differences of opinion in this process were resolved by discussion and agreement between the two researchers.
Literature quality evaluation
In this paper, two researchers used the Newcastle-Ottawa scale (NOS) to evaluate the quality of the included literature, including the selection of research subjects (4 points), the comparability between groups (2 points), and the measurement of exposure factors (3 points), a total of 9 points. Inconsistencies in the quality evaluation results between the two researchers were resolved through agreement after discussion.
Statistical method
This study used the Cochrane RevMan5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) for statistical analysis. The OR value and 95% CI were calculated using the Mantel-Haenszel statistical method to describe the effect quantity. OR was not adjusted for other factors. Cohort studies and case-control studies can be pooled. The Chi-square test was used to assess the heterogeneity between the included articles. When I2 corrected by degrees of freedom was >50% or P<0.1, it was considered that there was heterogeneity among the included studies, and a random effect model was used. Subgroup analysis was used to explore the causes of heterogeneity. When I2≤50% and P≥0.1, it was considered that there was no heterogeneity among the included literature, and the fixed effect model was used. A funnel plot was used to test for publication bias. Two-sided P<0.05 was considered to indicate statistical significance.
Results
Characteristics of the included literature
A total of 843 articles were retrieved from the above databases. According to the screening criteria, 834 articles were excluded, and a total of nine studies were included in this meta-analysis (6,11-18). The literature screening flow chart is shown in Figure 1. Among these nine articles, six were cohort studies, and three were case-control studies. The research objects of six cohort studies were not limited to patients with partial seizure type epilepsy, and those of the three case-control studies only included patients with partial seizure type epilepsy. One article was published in Chinese, and nine articles were in English. The basic information of the included literature and the NOS score are shown in Table 1.
Table 1
| Author and year | Study type | Epileptic seizure type | Language | No. of patients | No. of relapses | NOS |
|---|---|---|---|---|---|---|
| Karalok (6) 2020 | Cohort | All seizures types | English | 308 | 26 | 7 |
| Matricardi (15) 1989 | Cohort | Partial seizures | English | 458 | 84 | 7 |
| Olmez (11) 2009 | Case-control | All seizures types | English | 157 | 31 | 7 |
| Pavlović (16) 2011 | Cohort | Generalized seizures | English | 44 | 23 | 7 |
| Pavlović (17) 2012 | Cohort | Partial seizures | English | 52 | 19 | 7 |
| Qu (13) 2019 | Case-control | All seizures types | Chinese | 176 | 48 | 8 |
| Ramos-Lizana (14) 2010 | Cohort | All seizures types | English | 216 | 56 | 7 |
| Verrotti (18) 2000 | Case-control | Partial seizures | English | 84 | 24 | 7 |
| Verrotti (12) 2012 | Cohort | All seizures types | English | 168 | 30 | 8 |
NOS, Newcastle-Ottawa scale.
Overall analysis of EEG and recurrence after drug withdrawal
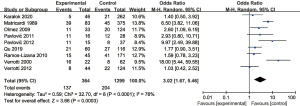
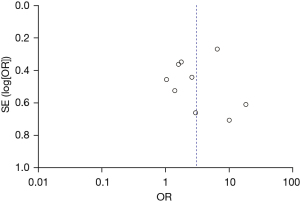
A total of 1,663 patients in the nine studies were included in our meta-analysis. There were 1,299 patients with normal EEG and 364 patients with abnormal EEG. Also, there were 204 cases of recurrence in patients with normal EEG and 137 in patients with abnormal EEG. Heterogeneity between the nine included articles was found (χ2=32.70, P<0.0001, I2=76%), and therefore, the random-effects model was used for combination. The analysis results showed that the recurrence rate of patients with abnormal EEG was OR =3.02 (95% CI: 1.67–5.46, Z=3.66, P=0.0003), as shown in Figure 2. The funnel diagram showed that the points were roughly symmetrically distributed in an inverted funnel shape, and there was no publication bias (Figure 3).
Subgroup analysis of EEG and recurrence after drug withdrawal
Subgroup analysis was carried out according to the different types of seizures, which were divided into two subgroups. One subgroup included three studies with partial seizure patients as the research object, and the other subgroup included six articles with all seizure types as the research object. There was no heterogeneity among the studies in the non-limited partial seizure subgroup (χ2=3.00, P=0.70, I2=0%), so the fixed-effects model was used for combination. The analysis results showed that compared to patients with abnormal and normal EEG, the recurrence rate after drug withdrawal was OR =1.70 (95% CI: 1.20–2.40, Z=3.02, P=0.003) (Figure 4).
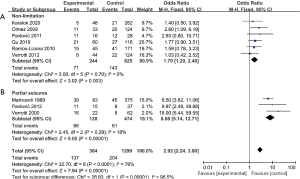
Furthermore, there was no heterogeneity among the studies involving some seizure types (χ2=2.45, P=0.29, I2=18%), so the fixed-effects model was used for combination. The analysis results illustrated that the recurrence rate of patients with abnormal EEG was OR =8.08 (95% CI: 5.14–12.71, Z=9.05, P<0.00001), as shown in Figure 4. The results were consistent between the two subgroups, and there was no heterogeneity among the subgroups.
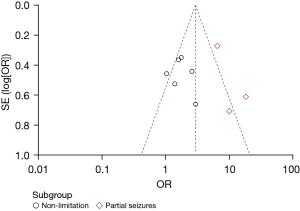
The subgroup analysis results were consistent with the overall results. The funnel chart of the subgroup analysis demonstrated that the literature on some seizure-type subgroups was biased towards positive results, and studies on non-limited partial seizure subgroups were biased towards negative results, as shown in Figure 5.
Discussion
Although a previous meta-analysis (19) confirmed that abnormal EEG after drug withdrawal was a risk factor for epilepsy recurrence, the research did not distinguish between children and adult subjects. There are differences in the brain structure and development between adults and children, as well as variations in seizure phenotype and pathological mechanism (19-21). The etiology of epilepsy in children is more complex than that in adults; the types of seizures are more diverse and are often atypical. Childhood seizures change with age. In terms of treatment, the functions of other organs of children’s nervous system diseases are not mature, improper drug selection is more prone to various adverse reactions, and AEDs need to be replaced with age (22-24). If childhood epilepsy is not treated in time, it eventually becomes adult epilepsy (25). The risk factors of epilepsy recurrence in children are complex, and the relationship between abnormal EEG after drug withdrawal and epilepsy recurrence in children is controversial.
A total of nine articles were included in this meta-analysis. The results showed that patients with abnormal EEG after drug withdrawal had a higher epilepsy recurrence rate than patients with normal EEG. Abnormal EEG after drug withdrawal is a risk factor for the recurrence of epilepsy in children. However, there was significant heterogeneity among the literature. This study identified the source of heterogeneity through subgroup analysis. There was no heterogeneity between the two subgroups grouped by different subjects. Therefore, we believe that heterogeneity came from the different research objects in the various studies. The research objects of three studies were only partial seizure patients, while those of the remaining six articles included patients with all seizure types, did not distinguish the seizure types, or only included patients with comprehensive seizure types. The analysis results of the two subgroups were in good agreement with the overall results, which supported the conclusion that the abnormal EEG after drug withdrawal was a risk factor for the epilepsy recurrence in children.
In the subgroup analysis, we found that the studies involving some seizure subgroups were biased towards positive results and considered that abnormal EEG was a risk factor for epilepsy recurrence in children. In the combined analysis, the OR value of the partial seizure subgroup was greater than that of the non-limited partial seizure subgroup. Our analysis suggests that this may be caused by the superposition of partial seizures and abnormal EEG. A study showed that seizure types are related to the recurrence of epilepsy in children, and partial seizures are risk factors for recurrence after drug withdrawal (6). The superposition of the two risk factors may lead to a significant increase in the risk of recurrence.
Our research also has some limitations that should be noted. Firstly, the sample size included in this study (1,663 patients in nine articles) is small. Therefore, it is necessary to expand the sample size in future research. Moreover, there was also no clear period of EEG examination after drug withdrawal in the included articles. Finally, some studies did not control the clinical baseline data of the observation and control groups. These deficiencies may affect the research results.
In conclusion, abnormal EEG after drug withdrawal is a risk factor for the recurrence of epilepsy in children. Therefore, children with epilepsy should be examined by EEG following drug withdrawal to evaluate the risk of epilepsy recurrence. For children with epilepsy and abnormal EEG after drug withdrawal, a more cautious drug withdrawal scheme and closer follow-up and monitoring are needed.
Acknowledgments
Funding: This project was supported by the Hainan Province Clinical Medical Center.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-206/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-206/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bastos F, Cross JH. Epilepsy. Handb Clin Neurol 2020;174:137-58. [Crossref] [PubMed]
- Devinsky O, Vezzani A, O'Brien TJ, et al. Epilepsy. Nat Rev Dis Primers 2018;4:18024. [Crossref] [PubMed]
- Stewart WA. Portraits of Children With Epilepsy. AMA J Ethics 2020;22:E544-549. [Crossref] [PubMed]
- Tanriverdi M, Mutluay FK, Tarakçi D, et al. The impact of epilepsy on preschool children and their families. Epilepsy Behav 2016;62:6-11. [Crossref] [PubMed]
- Frankel HG, Lam S. Seizure Outcomes of Epilepsy Surgery in Children. Pediatr Neurol Briefs 2021;35:2. [Crossref] [PubMed]
- Karalok ZS, Guven A, Öztürk Z, et al. Risk factors for recurrence after drug withdrawal in childhood epilepsy. Brain Dev 2020;42:35-40. [Crossref] [PubMed]
- Bouma PA, Peters AC, Brouwer OF. Long term course of childhood epilepsy following relapse after antiepileptic drug withdrawal. J Neurol Neurosurg Psychiatry 2002;72:507-10. [PubMed]
- Tan G, Li X, Niu R, et al. Microstructural features of the cerebral cortex: Implications for predicting epilepsy relapse after drug withdrawal. Brain Res 2021;1751:147200. [Crossref] [PubMed]
- Park S, Lee M. Prognostic Implications of Epilepsy Onset Age According to Relapse Pattern in Patients with Four-Year Remission. Diagnostics (Basel) 2020;10:1089. [Crossref] [PubMed]
- Callaghan B, Schlesinger M, Rodemer W, et al. Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia 2011;52:619-26. [Crossref] [PubMed]
- Olmez A, Arslan U, Turanli G, et al. Risk of recurrence after drug withdrawal in childhood epilepsy. Seizure 2009;18:251-6. [Crossref] [PubMed]
- Verrotti A, D'Egidio C, Agostinelli S, et al. Antiepileptic drug withdrawal in childhood epilepsy: what are the risk factors associated with seizure relapse? Eur J Paediatr Neurol 2012;16:599-604. [Crossref] [PubMed]
- Qu C, Zhu C. Factors of Recurrence of Pediatric Epilepsy after Withdrawal of Antiepileptic Drugs. Journal of Pediatric Pharmacy 2019;25:22-4.
- Ramos-Lizana J, Aguirre-Rodríguez J, Aguilera-López P, et al. Recurrence risk after withdrawal of antiepileptic drugs in children with epilepsy: a prospective study. Eur J Paediatr Neurol 2010;14:116-24. [Crossref] [PubMed]
- Matricardi M, Brinciotti M, Benedetti P. Outcome after discontinuation of antiepileptic drug therapy in children with epilepsy. Epilepsia 1989;30:582-9. [Crossref] [PubMed]
- Pavlović M, Jović N, Pekmezović T. Antiepileptic drugs withdrawal in patients with idiopathic generalized epilepsy. Seizure 2011;20:520-5. [Crossref] [PubMed]
- Pavlović M, Jović N, Pekmezović T. Withdrawal of antiepileptic drugs in young patients with cryptogenic focal epilepsies. Seizure 2012;21:431-6. [Crossref] [PubMed]
- Verrotti A, Morresi S, Cutarella R, et al. Predictive value of EEG monitoring during drug withdrawal in children with cryptogenic partial epilepsy. Neurophysiol Clin 2000;30:240-5. [Crossref] [PubMed]
- Yao J, Wang H, Xiao Z. Correlation between EEG during AED withdrawal and epilepsy recurrence: a meta-analysis. Neurol Sci 2019;40:1637-44. [Crossref] [PubMed]
- Besag FMC, Vasey MJ. Prodrome in epilepsy. Epilepsy Behav 2018;83:219-33. [Crossref] [PubMed]
- Fauser S, Tumani H. Epilepsy. Handb Clin Neurol 2017;146:259-66. [Crossref] [PubMed]
- Singhi P, Gupta A. Epilepsy in Children-Important Facets. Indian J Pediatr 2021;88:991-2. [Crossref] [PubMed]
- Jayalakshmi S, Vooturi S, Gupta S, et al. Epilepsy surgery in children. Neurol India 2017;65:485-92. [Crossref] [PubMed]
- Bello-Espinosa LE, Olavarria G. Epilepsy Surgery in Children. Pediatr Clin North Am 2021;68:845-56. [Crossref] [PubMed]
- Matern TS, DeCarlo R, Ciliberto MA, et al. Palliative Epilepsy Surgery Procedures in Children. Semin Pediatr Neurol 2021;39:100912. [Crossref] [PubMed]



