
A juvenile murine model with chronic lung inflammation induced by repeated intratracheal instillation of lipopolysaccharides: a versatile and replicable model
Introduction
Chronic lung diseases (CLDs), characterizing prolonged course and impaired lung function, have great impact on patients’ quality of life. In recent years, incidences of CLD with high disability and fatality rates, including interstitial lung disease, bronchiolitis obliterans, primary ciliary dyskinesia, cystic fibrosis and others, have increased in children (1). Apart from primary CLD, secondary CLD is mainly caused by infection, transplantation, toxicants or connective tissue disorders, in which post-infection CLD has become a concern for pediatricians. A meta-analysis revealed that 17% of children with severe pneumonia developed CLD as long-term sequelae, such as bronchiectasis and chronic bronchitis (2). Due to abnormal structures of respiratory tracts and mucus obstruction, children with CLD are more likely to suffer from respiratory infections than healthy children, which have more severe and longer courses accompanied by decreased baseline lung function (3). Recurrent lower respiratory tract infection (RLRI) (4) often occurs in CLD patients. For post-infection CLD, respiratory tract infection and CLD reinforce each other as vicious cycles. RLRI or chronic infection leads to chronic airway inflammatory which provokes persistent lung injury, irreversible structural and lung function damage. Chronic lung inflammation plays a key role in this deterioration process. However, there are some gaps in current studies on CLD and RLRI. (I) Most common animal models of CLD are chemical-induced, such as diacetyl-induced bronchiolitis obliterans (5), cigarette smoke-induced chronic bronchitis (6) and bleomycin-induced pulmonary fibrosis (7), which could not explore the effects of chronic lung inflammation under repeated infections. (II) Instead of studying how chronic inflammation itself alter pulmonary structures, current RLRI or chronic infection animal models focus on specific bacteria. To investigate structural and functional change in children’s lungs under sustained and intense inflammation state, a chronic lung inflammation animal model which can recapitulate such real-world situation is in urgent need.
With impaired epithelium and abnormal pulmonary immune status, bacterial colonization often induces RLRI or chronic lung infection in CLD patients (8). Gram-negative (G-) bacteria, such as Haemophilus influenzae and Pseudomonas aeruginosa (PA), are commonly colonized in the airway of CLD patients, among which PA plays important role throughout the course of most cystic fibrosis patients (9). Lipopolysaccharides (LPS), as a pro-inflammatory glycolipid component on the cell wall of G- bacteria (10), are major causative agents during lung injury and pneumonia (11). As purification and production procedure standardized, virulence heterogeneity of LPS obtained by the same manufacturer, extraction method and bacteria source greatly decreased. Therefore, by ensuring relatively stable model quality, LPS have been widely used to induce inflammation in vitro and in vivo (12), especially a common stimulus for pulmonary inflammatory disease models.
In order to attain chronic lung inflammation in animal models, methods of LPS administration (12) primarily include nebulization and intratracheal instillation. To reproduce RLRI inducing persistent pulmonary inflammation in mice, LPS must access lower respiratory tract, thus intratracheal instillation is preferred. In current models of LPS-induced lung inflammation, the concentrations of LPS are either too high or too low. In LPS-induced acute lung injury models (13), to achieve diffuse alveolar damage, concentration of LPS is higher than 2 mg/kg and animals are usually sacrificed within 72 hours after the instillation. While in LPS-induced chronic bronchitis and chronic obstructive pulmonary diseases models, low concentration of LPS, generally less than 0.5 mg/kg are given for more than 10 weeks to ensure the animals could survive until airway structure changes (14,15). In purpose of recapitulating persistent high-intensity lung inflammation caused by RLRI or chronic infection, we hypothesized that slightly increased the concentration of LPS could trigger greater lung lesions and chronic structural-changed lung inflammation may occur after repeated instillation. Here, we reported a juvenile murine model with chronic lung inflammation induced by repeated intratracheal instillations of Pseudomonas aeruginosa LPS. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-44/rc).
Methods
Animals
Four-week-old C57BL/6N female mice (weight 13–15 g) were obtained from Charles River Breeding Laboratories (Beijing, China). Mice were housed and bred under standard conditions of care. Experiments were performed under a project license (No. 2021019) granted by the Animal Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, in compliance with Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
Experimental protocol
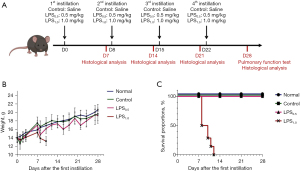
Mice were randomly divided into 4 groups, including LPS0.5 group (n=15), LPS1.0 group (n=15), Control group (n=15) and Normal group (n=15). In order to induce chronic pulmonary inflammation, mice in LPS groups were repeatedly challenged with LPS (Pseudomonas aeruginosa 10, L9143, Sigma Chemical Co.) once a week for 4 weeks by intratracheal instillation. Mice in LPS0.5 group and LPS1.0 group were instilled intratracheally with 0.5 mg/kg LPS and 1.0 mg/kg LPS respectively. Mice in control group were instilled intratracheally with LPS-free sterile 0.9% NaCl, whereas normal group received no treatment. The administrated volume of LPS and saline were 50 µL, respectively. Mice were anesthetized by intraperitoneal injection of Avertin. A volume of 50 µL LPS or saline was instilled intratracheally via a canule, followed by 0.1 mL of air. After intratracheal treatment, the mice were kept in an upright position for 10 min to allow sufficient spreading of the fluid throughout the lungs. In order to explore the appropriate concentration of LPS, mice were killed on day 7, 14, 21, and 28 after the first instillation to observe the pathological changes of lung tissues. On day 28, 6 mice from each group were taken for lung function testing (Figure 1A).
Histological analysis
Lung tissue was inflated with 10% phosphate-buffered formalin (pH 7.4) at a pressure of 20 cmH2O through the trachea for 15 minutes and subsequently fixed in formalin for 24 h. After paraffin embedding, 4-µm sections were cut and stained with hematoxylin/eosin (H&E) to evaluate general morphology.
To quantify the extent of inflammatory infiltration and tissue damage, we developed a modified lung inflammation scoring system (Table 1) based on previous studies (16-18). Whole-lung sections were scored by two independent observers who were blinded for groups. Degree of inflammatory cell infiltration and destruction of parenchyma were assessed in perivascular regions, peribronchial regions, alveolar septa and lumens. Modified lung inflammation score was expressed as the sum of scores for each parameter. Scores of mice from the same group were pooled and calculated as mean ± standard error of the mean (SEM).
Table 1
| Regions | Parameters | Scores | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Perivascular | (I) Inflammatory cell infiltration | Absent | Mild | Moderate | Severe |
| (II) Destruction of parenchyma | Absent | Mild | Moderate | Severe | |
| Peribronchial | (I) Inflammatory cell infiltration | Absent | Mild | Moderate | Severe |
| (II) Destruction of parenchyma | Absent | Mild | Moderate | Severe | |
| Alveolar | (I) Inflammatory cell infiltration | Absent | Mild | Moderate | Severe |
| (II) Compressed alveolar lumens | Absent | Mild | Moderate | Severe | |
| (III)Alveolar septal thickening | Absent | Mild | Moderate | Severe | |
Lung function measurement
FlexiVentTM system was used for lung function measurement. First, calibrate the pulmonary function meter and set up parameters. After intraperitoneal injection of 1.2% avertin to anesthetize the mouse, cut the skin at the thyroid cartilage, bluntly separate the muscles to expose the trachea. Then cut a small opening under the thyroid cartilage and insert canule into trachea. After finishing intubation, move the mouse into plethysmography chamber, intraperitoneally inject muscle relaxant vecuronium (0.3 mL/kg), connect the system and complete lung function assessment.
Statistical analysis
The unpaired, 2-tailed t-test was used to determine whether the differences in the lung function parameters between Normal group, Control group and LPS0.5 group was statistically significant. Comparison and statistical analysis of modified lung inflammation score between different groups were performed with the Mann-Whitney U test. Tests were performed with GraphPad Prism. P values of 0.01–0.05, 0.001–0.01, and 0.0001–0.001 were considered statistically significant, very significant, and extremely significant, respectively.
Results
Weight change and survival proportions
During the first instillation (D0), mice in the Control group were short of breath after control instillation, which recovered after 5 minutes. They woke up after an average of 20 minutes and their actions and reactions recovered as before instillation. In LPS0.5 and LPS1.0 group, shortness of breath was more pronounced which took averagely 40 minutes for them to recover. On the first day after the first instillation (D1), mice in the LPS group lost an average of 10% in weight. Among them, weight of mice in LPS0.5 group gradually recovered as Normal group since D3, while mice in LPS1.0 group slowly recovered since D4 (Figure 1B). During the second instillation (D8), mice in LPS1.0 group had poorer tolerance to anesthetics compared with LPS0.5 group. In LPS1.0 group, a mouse died after anesthesia and 7 mice died quickly due to dyspnea after instillation. The remaining mice in LPS1.0 group died on D9, D10, and D11 (1, 2, and 3 days after the second instillation respectively). All mice in LPS1.0 group died before the third instillation on D15 (Figure 1C). No death after instillation was observed in Control and LPS0.5 group (Figure 1C).
Dynamic change and characteristics of chronic inflammation during 4 weeks of LPS exposure
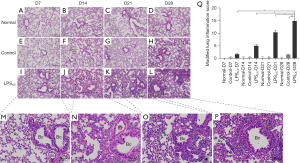
For mice in in normal (Figure 2A-2D) and control group (Figure 2E-2H), no obvious inflammation was observed in lung tissues throughout the experiment. In mice of LPS0.5 group: (I) 7 days after the first instillation (D7, Figure 2I), few mononuclear cells infiltrated around part of the bronchus, with intact airway epithelium. (II) 7 days after the second instillation (D14, Figure 2J), bronchial epithelium thickened with a few neutrophils and mononuclear cells infiltration, while no exudates in the alveoli were observed. (III) 7 days after the third instillation (D21, Figure 2K), small amounts of bronchial epithelial cells fell off with few mononuclear cells infiltrating around bronchus and severer lymphocytic infiltration around parabronchial vessels. Local alveolar wall slightly thickened with few mononuclear cells infiltrated while no exudate in the alveolar cavity were observed. (IV) 7 days after the third instillation (D28, Figure 2L), most bronchus and parabronchial vessels are wrapped by lymphocytic aggregation. While alveolar wall thickened with focal infiltration of mononuclear cells and connective tissue hyperplasia, alveolar cavities are compressed and narrowed without exudates in the cavity. Under high-magnification view (Figure 2M-2P), we found that agranulocytes constituted the mononuclear-cell infiltration and lymphocytic aggregation in LPS0.5 group, which mainly consisted of monocytes, with oval, indented or C-shaped nucleus, and lymphocytes, with rather spherical nucleus. Comparisons of modified lung inflammation scores between mice from different groups (Figure 2Q) showed that, there were statistically significant differences between LPS0.5 group on D28 and on D7 (P<0.05), D14 (P<0.05), D21 (P<0.05) and Control group on D28 (P<0.01). These indicated that mice of LPS0.5 group on D28 had more intense inflammatory pulmonary damage.
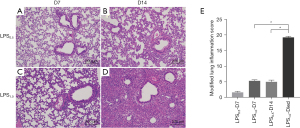
All mice in LPS1.0 group died before the third instillation (D15) and their lung tissues were taken for morphological analysis. Compared with LPS0.5 group (Figure 3A,3B), mice in LPS1.0 group (Figure 3C,3D): (I) on D7, more mononuclear cells were observed around parabronchial vessels, while alveolar walls also thickened with mononuclear-cell infiltration. (II) For mice that died before the third instillation, small amounts of epithelial cells fell off and alveolar cavity was severely compressed by excessively infiltration of neutrophils and mononuclear cells. Modified lung inflammation scores of LPS1.0 group (Figure 3E) on D14 were significantly higher than D7 (P<0.05) and LPS0.5 group on D14 (P<0.05), revealing that mice in LPS1.0 group might die of alveolar gas-exchange dysfunction, as consequences of excessive inflammatory infiltration.
Ventilatory dysfunction developed after 4 weeks of LPS exposure
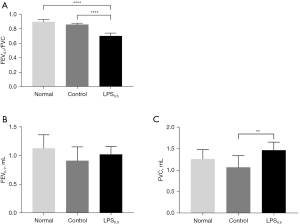
Forced vital capacity (FVC) and forced expiratory volume in 0.1 second (FEV0.1) were measured (Table 2 and Figure 4) to evaluate lung function of mice in normal group (n=6), control group (n=6) and LPS0.5 group (n=6) on D28 (7 days after the fourth instillation). No statistical differences in FEV0.1 were observed between three groups (Figure 4B) and FVC of LPS0.5 group was higher than that of Control group (Figure 4C). Whereas, FEV0.1/FVC was significantly lower than Control and Normal group (Figure 4A), suggesting ventilatory dysfunction developed after repeatedly intratracheal instillation once a week for 4 weeks.
Table 2
| Group | FEV0.1 (mL, mean ± SD) | FVC (mL, mean ± SD) | FEV0.1/FVC (mean ± SD) |
|---|---|---|---|
| Normal (n=6) | 1.133±0.233 | 1.261±0.216 | 0.896±0.034 |
| Control (n=6) | 0.915±0.243 | 1.066±0.273 | 0.858±0.018 |
| LPS0.5 (n=6) | 1.025±0.140 | 1.462±0.191 | 0.701±0.040 |
LPS, lipopolysaccharides; FEV0.1, forced expiratory volume in 0.1 second; FVC, forced vital capacity; SD, standard deviation.
Pathologic findings of chronic inflammatory cell infiltration, lung parenchyma injury and decreased lung function were observed in all mice in LPS0.5 group on D28 (n=6), reflecting that the success rate of this LPS-induced chronic lung inflammation murine model was 100%.
Discussion
Previous studies (19,20) showed that intratracheal instillation 2 mg/kg of LPS can cause acute lung injury in adult C57BL/6 mice. Acute inflammation peaks 2 days after instillation, and then significantly alleviated 7 days after instillation (19). In the commonly used chronic bronchitis murine model induced by aerosolized low-concentration LPS, infiltration of neutrophils that appeared quickly post-nebulization resolved 1 week after nebulization termination, indicating that acute inflammation began to recover 1 week after LPS exposure (14). Therefore, this study chose 7 days as the instillation interval. In order to ensure the survival rate of mice after repeated LPS instillation, two concentrations of LPS (1.0 and 0.5 mg/kg) were used in this study.
Features of chronic lung inflammation are: (I) clumps of chronic inflammatory cells (mainly lymphocytes and monocytes); (II) destruction of lung parenchyma; (III) connective tissue proliferation and repair (21). In this study, repeated intratracheal instillation of 0.5 mg/kg LPS showed the accumulation process of chronic inflammatory cells in the lung parenchyma, which also infiltrate bronchus and parabronchial vessels. With lung parenchyma excessively infiltrated by inflammatory cells, alveolar cavities are narrowed. Also, although fibrosis is not obvious in this model, a certain extent of connective tissue hyperplasia can be seen in the lung parenchyma. Similar morphological characteristics were also observed in previous studies (14,22), which demand more than 10 weeks to attain chronic lung inflammation. Cavagna et al. established a rabbit model of chronic bronchitis by nebulizing LPS dissolved in saline solution. After nebulization for 20 weeks, bronchitis and bronchiolitis manifested as bronchial epithelial cells shed off, endobronchial secretions increased, inflammatory cells infiltrated around bronchus and alveolar walls, accompanied with elevated airway resistance and reduced lung compliance (23). Vernooy et al. instilled 5 µg of LPS into the trachea of 12-week-old Swiss mice twice a week. After 12 weeks, inflammatory cells infiltrated the bronchus, bronchioles, and large vessels. Alveolar infiltration of monocytes and lymphocytic infiltration in bronchial epithelium resulted in local bronchiolitis (14). After aerosol inhalation of LPS for 6 weeks, both wild-type and Cftr-/- mice exhibited expansion of the distal bronchiolar alveolar cavity and destruction of alveolar structure (15). At present, studies on animal models of chronic lung inflammation via repeated instillation of low-concentration LPS into the trachea require at least 6 weeks (15,22). In order to simulate impact of RLRI and subsequent chronic lung inflammation, this study aim to seek the appropriate concentration of LPS which ensure long-term survival rate and maintain the intensity of chronic lung inflammation, which could reduce time required for chronic inflammation formation. Moreover, the significantly reduced FEV0.1/FVC in LPS0.5 group suggests that repeated LPS instillation for 4 weeks can make enough structural damages to impair lung function. However, fibrotic remodeling was not observed in our study.
In conclusion, intratracheal instillation of 0.5 mg/kg LPS once a week for 4 weeks can cause chronic lung inflammation in young mice and we expect this model for further use in mechanism exploration and drug development.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81770063) and Zhongnanshan Medical Foundation of Guangdong Province (grant number 202102010343) and the Science and Technology Program of Guangzhou (grant number 202102010276).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-44/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-44/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-44/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2021019) granted by the Animal Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, in compliance with Regulations for The Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jobe AH, Tibboel D. Update in pediatric lung disease 2013. Am J Respir Crit Care Med 2014;189:1031-6. [Crossref] [PubMed]
- Edmond K, Scott S, Korczak V, et al. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS One 2012;7:e31239. [Crossref] [PubMed]
- Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014;384:691-702. [Crossref] [PubMed]
- de Benedictis FM, Bush A. Recurrent lower respiratory tract infections in children. BMJ 2018;362:k2698. [Crossref] [PubMed]
- Palmer SM, Flake GP, Kelly FL, et al. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One 2011;6:e17644. [Crossref] [PubMed]
- Tanner L, Single AB. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J Innate Immun 2020;12:203-25. [Crossref] [PubMed]
- Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152-60. [Crossref] [PubMed]
- Chang AB, Redding GJ, Everard ML. Chronic wet cough: Protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol 2008;43:519-31. [Crossref] [PubMed]
- Garcia-Clemente M, de la Rosa D, Máiz L, et al. Impact of Pseudomonas aeruginosa Infection on Patients with Chronic Inflammatory Airway Diseases. J Clin Med 2020;9:3800. [Crossref] [PubMed]
- Cavaillon JM. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018;149:45-53. [Crossref] [PubMed]
- Chakraborty A, Boer JC, Selomulya C, et al. Insights into endotoxin-mediated lung inflammation and future treatment strategies. Expert Rev Respir Med 2018;12:941-55. [Crossref] [PubMed]
- Knapp S. LPS and bacterial lung inflammation models. Drug Discovery Today: Disease Models 2009;6:113-8. [Crossref]
- Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 2012;91:590-601. [Crossref] [PubMed]
- Vernooy JH, Dentener MA, van Suylen RJ, et al. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol 2002;26:152-9. [Crossref] [PubMed]
- Bruscia EM, Zhang PX, Barone C, et al. Increased susceptibility of Cftr-/- mice to LPS-induced lung remodeling. Am J Physiol Lung Cell Mol Physiol 2016;310:L711-9. [Crossref] [PubMed]
- Knapp S, Florquin S, Golenbock DT, et al. Pulmonary lipopolysaccharide (LPS)-binding protein inhibits the LPS-induced lung inflammation in vivo. J Immunol 2006;176:3189-95. [Crossref] [PubMed]
- Kajon AE, Gigliotti AP, Harrod KS. Acute inflammatory response and remodeling of airway epithelium after subspecies B1 human adenovirus infection of the mouse lower respiratory tract. J Med Virol 2003;71:233-44. [Crossref] [PubMed]
- Patel BV, Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J 2012;39:1162-70. [Crossref] [PubMed]
- Maron-Gutierrez T, Silva JD, Asensi KD, et al. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med 2013;41:e319-33. [Crossref] [PubMed]
- Fragoulis A, Biller K, Fragoulis S, et al. Reference Gene Selection for Gene Expression Analyses in Mouse Models of Acute Lung Injury. Int J Mol Sci 2021;22:7853. [Crossref] [PubMed]
- Vinay Kumar AA, Jon Aster. Robbins Basic Pathology. 10th edition. Philadelphia: Elsevier; 2017.
- Corbel M, Theret N, Caulet-Maugendre S, et al. Repeated endotoxin exposure induces interstitial fibrosis associated with enhanced gelatinase (MMP-2 and MMP-9) activity. Inflamm Res 2001;50:129-35. [Crossref] [PubMed]
- Cavagna G, Foà V, Vigliani EC. Effects in man and rabbits of inhalation of cotton dust or extracts and purified endotoxins. Br J Ind Med 1969;26:314-21. [Crossref] [PubMed]


