
Management of pancreatic kaposiform hemangioendothelioma with sirolimus in a pediatric patient: a case report and literature review
Introduction
Kaposiform hemangioendothelioma (KHE) is a rare locally aggressive vascular tumor categorized as an intermediate grade malignant tumor according to the recent World Health Organization (WHO) classification (1,2). Although typically considered to affect infants and children, more and more adult cases are being reported (1). Given that no guidelines are available for the treatment of KHE, its management is currently based on expert opinion and clinical experience (3,4). Characterized by thrombocytopenia and coagulopathy, Kasabach-Merritt phenomenon (KMP) is a complication of KHE with high mortality and various drug responses, for which treatment decisions are difficult to make (5-8). Retroperitoneal KHE, which may cause hemorrhage of the digestive tract, jaundice, and obstruction, is relatively rare compared to cutaneous KHE (5,9), and pancreatic KHE is even more rare, with only sporadic cases having been reported so far. Herein, we report a case of pancreatic KHE with obstructive jaundice, which was treated successfully with oral sirolimus instead of radical surgery. Additionally, a literature review was conducted on pancreatic KHE to summarize clinical experiences and individual treatments. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-38/rc).
Case presentation
A 10-month-old Chinese male infant was referred to our hospital due to obstructive jaundice and hospitalized for further evaluation in the Department of Medicine of Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China. The patient showed no signs of fever, abdominal pain, or distention, and had a good appetite and normal feces. A detailed consultation revealed an uneventful history. Physical examination showed cutaneous and conjunctival jaundice without hepatomegaly. Laboratory tests showed slightly elevated liver enzymes [alanine aminotransferase (ALT) 147 U/L, aspartate aminotransferase (AST) 65 U/L, gamma-glutamyl transferase (GGT) 144 U/L] and obstructive jaundice with total bilirubin of 71.7 µmol/L and direct bilirubin of 57.9 µmol/L. Further investigations were performed to exclude other factors that could lead to obstructive jaundice. In particular, blood tests for hepatopathy pathogens such as hepatovirus and distomiasis were all found negative. Pathological findings from metabolic testing showed no amino acids and acylcarnitines in the blood or organic acids in the urine. It is worth mentioning that KPM was ruled out based on a normal platelet count, normal clotting profile, and no clinical manifestation of bleeding tendency during the hospitalization.
Ultrasonic (US) examination revealed slight dilatation of the bile duct without a pancreatic mass during the first outpatient visit prior to hospitalization. However, a re-evaluation using ultrasound 2 weeks after hospitalization revealed a slightly hyperechoic mass in the pancreatic head.
The department of surgery was consulted after 3 weeks of conservative treatment, during which direct bilirubin had increased from 57.9 to 128.5 µmol/L.
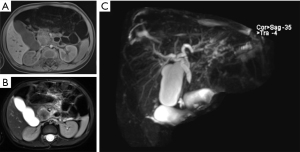
Magnetic resonance cholangiopancreatography (MRCP) and computed tomography (CT) with contrast was advised for a more comprehensive assessment of the mass. The MRCP imaging showed a 3.0×2.8×2.4 cm mass in the uncinate process of the pancreatic head, presenting an equal-high signal on T1-weighted imaging (T1WI) (Figure 1A) and a low-equal signal on T2-weighted imaging (T2WI) (Figure 1B), which caused a complete cutoff of the common bile duct and dilatation of the proximal bile duct (Figure 1C). A CT scan of the abdomen and pelvis with intravenous contrast agent showed an ill-defined, enhanced mass in the head of the pancreas with a complete cutoff of the common bile duct (Figure 2A-2C).
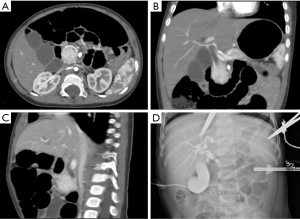
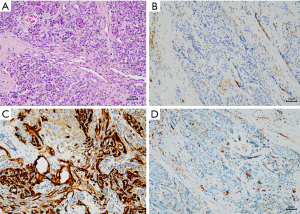
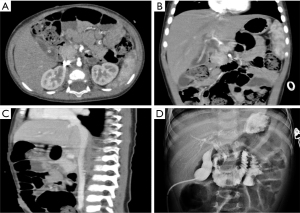
A laparoscopic biopsy of the tumor was performed after a pancreatic mass had been confirmed. A cholangiography of the gallbladder confirmed our finding of a dark-purple tumor in the pancreatic head surrounding the common bile duct, which caused a total obstruction of the common bile duct (Figure 2D). Fast-frozen pathology during the surgery revealed a vascular tumor, most likely KHE. Our surgical team decided to perform a multipunch biopsy and cholecystostomy to drain the bile rather than performing a radical Whipple operation before the diagnosis was finally made. After KHE had been confirmed by pathological findings (Figure 3), oral sirolimus 0.8 mg/m2 twice daily was administered at a steady serum concentration of 5–15 ng/mL. At 3 weeks after the sirolimus treatment, bilirubin decreased to a normal range, and the drainage of cholecystostomy decreased from more than 100 mL daily to null daily. Reassessment with an enhanced CT scan showed shrinkage of the lesion and a lower degree of enhancement compared with previous imaging (Figure 4A-4C). A second surgery was performed 5 weeks after the first surgery. Cholangiography through cholecystostomy showed a clear bile duct (Figure 4D), and as such the drainage was removed. The patient was discharged the day after the surgery with oral sirolimus treatment. During a year of follow-up, the patient showed no clinical manifestations such as jaundice or purpura, and the US examination showed no mass in the pancreas and no obstruction of the bile duct. Moreover, D-dimer, fibrinogen, liver function, and platelet count remained normal. To date, the patient is still undergoing sirolimus treatment and is being followed up regarding clinical manifestations, radiology examinations, serum concentrations of sirolimus, and side-effects.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient’s parents for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Pancreatic tumors are relatively rare in children, especially so in neonates and infants (10). However, pancreatic KHE often preferentially affects infants, as shown in our series of reported cases, where all pediatric cases were younger than 2 years old (Table 1). Diagnosis during the early infant period means that KHE may have started before birth. However, no prenatal diagnosis has been reported in the literature.
Table 1
| Author | Age | Manifestation | With KMP or not | Tumor location (maximum diameter in size) | Diagnosis method | Initial treatment | Secondary treatment | Surgery/interventional treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Mathew 2019 (11) | 8 months | Obstructive jaundice | Yes | Pancreatic head with hepatic portal involved (2.8 cm) | Histopathology | IFN-α | – | Laparotomy biopsy | Deceased |
| Yao 2020 (9) | 50 days | Jaundice/cutaneous petechiae | Yes | Pancreatic head with hepatic portal involved (4.9 cm) | Clinical# | Steroids and VCR | Sirolimus | – | Tumor shrinkage |
| Yao 2020 (9) | 80 days | Jaundice/cutaneous petechiae | Yes | Pancreatic head with hepatic portal involved (9.9 cm) | Clinical# | Steroids and VCR | Sirolimus | Percutaneous biliary diversion | Tumor shrinkage |
| Yao 2020 (9) | 73 days | Jaundice/cutaneous petechiae | Yes | Pancreatic head (5.6 cm) | Clinical# | Steroids and VCR | Sirolimus | – | Tumor shrinkage |
| Triana 2017 (12) | 4 months | Obstructive jaundice | Yes | Pancreatic head (3.3 cm) | Histopathology | Sirolimus | VAT | Laparotomy biopsy/percutaneous biliary diversion/biliary stent/open hepaticojejunostomy with Roux-en-Y reconstruction (with stent removed) | Tumor shrinkage with fibrosis |
| Leung 2014 (13) | 7 days | Bile-stained vomiting/thrombocytopenia | No | Pancreatic head (5 cm) | Histopathology | Surgery | – | Whipple operation | Alive and disease free |
| Chundriger 2021 (1) | 18 years | Obstructive jaundice | No | Pancreatic head (1.5 cm) | Histopathology | Sirolimus | – | Biopsy | Alive and disease free |
| Wang 2017 (14) | 16 months | Obstructive jaundice | No | Pancreatic head (2.2 cm) | Histopathology | Sirolimus (0.04 mg/kg) | – | End-to-side jejunojejunostomy and chole-cystojejunostomy | Alive and disease free |
| Kim 2021 (10) | 28 days | White stool jaundice |
No | Uncinate process (2.3 cm) | Histopathology | Surgery | IFN-α therapy for 4 months | Pylorus-preserving pancreaticoduodenectomy | Alive and disease free |
#, clinical diagnosis means a diagnosis by clinical evidence such as imaging rather than a histopathological result in diagnosing KHE. KHE, kaposiform hemangioendothelioma; KMP, Kasabach-Merritt phenomenon; IFN-α, interferon alpha; VCR, vincristine; VAT, vincristine/aspirine/ticlopidine.
Compared to cutaneous KHE, pancreatic KHE can result in obstructive jaundice, bowel obstruction, and cutaneous petechiae according to our series of reported cases. Obstructive jaundice caused by obstruction of the bile duct is the most common manifestation accounting for 9 out of 10 cases (with our case included) (Table 1). All the reported cases of pancreatic KHE in our case series involved the pancreatic head (Table 1). There is no theory or hypothesis describing the relationship between this lesion location in terms of the etiology or the development of the tumor, and more research is required to explain this phenomenon.
Ryu et al. (15) summarized the imaging features of CT and magnetic resonance (MR) imaging in KHE and described the radiological characteristics as infiltrative lesions with solid, well-defined central regions compared to other vascular lesions or sarcomas. Unenhanced CT scans of KHE mostly exhibited homogeneous iso-attenuation similar to adjacent muscle, while enhanced CT exhibited heterogeneous enhancement ranging from mild to intense in terms of the degree of enhancement. Unenhanced MR images showed heterogeneous and hyperintense enhancement relative to muscles on T2WI, while enhanced MR images exhibited mostly intense heterogeneous enhancement (15). The heterogeneous enhancement was likely associated with the pathological nature of vascular channels and thin-walled lymphatic vessels in KHE (11,15). For these radiological characteristics of KHE, Yao et al. (9) reported 3 cases clinically diagnosed as KHE based on clinical manifestations and imaging results, and these authors reported initiating sirolimus therapy without histologic diagnosis. Although good treatment results with no evidence of tumor recurrence and normal platelet count and liver function were achieved in this case series, no clinical guidelines are available to support radiological diagnosis, and as such, initiation of therapy warrants caution.
Histopathological evidence is the gold standard for diagnosis and individualized treatment. The typical histopathological manifestation of KHE is infiltrating, defined, rounded, and confluent nodules composed of spindle endothelial cells, which align to form malformed lymphatic channels and slit-like vascular lumina. These lumina, which contain erythrocytes, platelet thrombi, eosinophilic hyaline bodies, and extravasation of hemosiderin deposits are responsible for life-threatening thrombocytopenic coagulopathy, which is called KMP (3,11,16). Immunohistochemical staining shows that endothelial cells in KHE lesions are positive for the vascular endothelial markers CD31 and CD34 and lymphatic endothelial markers VEGFR-3, D2-40, LYVE-1, and Prox-1, but negative for Glut-1 and HHV-8 staining (3,16).
Kasabach and Merritt first described KMP in 1940 as purpura in patients with capillary hemangioma and clinically characterized by purpuric, hot, swollen, and enlarged KHE lesions with thrombocytopenia, coagulopathy, and hypofibrinogenemia with elevated D-dimer in laboratory tests (3,5,6,17). A hypothesis was that altered endothelial cells in KHE lesions triggered platelet trapping in the lesion, which resulted in a coagulation cascade and eventually led to KMP (3,16). The literature reports that KMP can occur in more than 70% of KHE patients and is more common in cutaneous lesions with a diameter greater than 8 cm or lesions with involvement of the retroperitoneum or visceral organs (5,11). With mortality reported as high as 10–20% (6,7), patients with KMP also showed a wide variation in response to steroid therapy and other first-line drugs for KHE (8). In recent years, sirolimus in combination with steroids has been recommended as one of the first-line therapies with good clinical results (18,19). In our series of cases, 5 out of 10 cases of pancreatic KHE were affected by KMP (Table 1), which is a relatively low proportion compared with that reported in other studies. Pancreatic KHE may have early symptoms such as jaundice leading to early diagnosis, which eventually leads to early treatment prior to the occurrence of complicating KMP.
Radical surgery to remove this vascular tumor still seems to be a reasonable treatment of choice. Leung et al. (13) reported a newborn male baby manifesting duodenal obstruction and thrombocytopenia. Although frozen section pathologic examination during the surgery revealed a vascular tumor, likely KHE, the surgical team decided to remove the tumor via Whipple operation, and the baby boy had recovered and was in a good condition during follow-up. In our case, our surgical team had previously planned for radical surgery, likely a Whipple operation. However, the long-term effects of Whipple operations on newborns are still uncertain and require more attention. Our concerns about patients’ long-term quality of life prompted us to postpone surgery and wait for histological results.
Another clinical option are pharmaceutical interventions, but no optimal treatment strategy has been fully accepted for KHE, and variable responses to vincristine, propranolol, corticosteroids, and interferon have been reported in the literature (7,8). Conversely, sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR) exerts evidentially-substantiated effects on the PI3K-Akt-mTOR pathway, which is widely involved in cell metabolism, growth, and proliferation (20,21). In animal models, the anti-angiogenic and anti-lymphangiogenic effects of mTOR in vascular tumors including KHE have been supported (22,23), and this preclinical testing provides the evidence for clinical application of sirolimus. Following clinical applications over a number of years (1,9-11,14), sirolimus has recently been recommended as a new treatment for KHE with or without KMP. In a study of sirolimus with more than 2-year follow-up, 73% (19/26) of patients showed shrinkage with single sirolimus treatment, while the remaining cases received sirolimus combined with vincristine and steroids and showed shrunken tumors (24). Moreover, the platelet and hemoglobin levels of all the cases including 25 patients with KMP reached normal levels within several weeks following treatment (24). In our case series, tumor shrinkage post sirolimus therapy was seen in 5 out of 6 patients including 3 cases showing no initial response to steroids and vincristine. Wang et al. (24) also summarized their experience with using sirolimus for the treatment of KHE as follows. First, the therapy should begin at a dose of 0.8 mg/m2 twice a day, and the preferred drug concentration in plasma should be maintained within 10–15 ng/mL. Second, plasma levels tend to stabilize after 1 month. Finally, sirolimus should be discontinued after approximately 24 months and gradually tapered during the final 6 months of treatment, and after the serum level reaches <5 ng/mL, the treatment could be discontinued.
Despite the good clinical results achieved so far with its application, long-term follow-up data on sirolimus therapy in KHE are not yet available. Some articles that have documented unsatisfactory results or adverse effects with sirolimus therapy deserve attention. Triana et al. (12) reported the case of a 4-month-old infant with KHE treated with sirolimus. The tumor disappeared leaving fibrosis and dilatation of the biliary tract, which required hepaticojejunostomy 6 months after the treatment. Ying et al. (25) reported 2 infant deaths because of infection potentially caused by sirolimus, demonstrating a risk of infection with sirolimus therapy especial in infant cases. More data are needed on the long-term effects of sirolimus in terms of clinical results, tumor recurrence, and adverse effects.
Although only a few case reports were available, we summarized the clinical manifestations, treatment options, and results of pancreatic KHE to share our experiences and provide guidance in the management of pancreatic KHE based on the limited existing literature. These diagnostic and treatment experiences are likely to benefit other pediatric surgeons, when they first encounter this rare disease.
In conclusion, pancreatic KHE is a rare location of this aggressive vascular tumor. Diagnosis is based on histological findings, and the therapy should be multidisciplinary and individualized. Although sirolimus has been very successful in the treatment of KHE even without a radical surgery until now, the possible risk of tumor recurrence and adverse effects warrants some caution.
Acknowledgments
The authors would like to thank the patient and his family for their consent to publish this report. The authors would also like to thank B. Meiser and J. Jones from AME language editing service for their language advices on our work.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 82170528) and the Natural Science Foundation of Guangdong Province (No. 2022A1515012254 and No. 2018A030313570).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-38/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient’s parents for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chundriger Q, Tariq MU, Abdul-Ghafar J, et al. Kaposiform Hemangioendothelioma: clinicopathological characteristics of 8 cases of a rare vascular tumor and review of literature. Diagn Pathol 2021;16:23. [Crossref] [PubMed]
- Mahajan P, Margolin J, Iacobas I. Kasabach-Merritt Phenomenon: Classic Presentation and Management Options. Clin Med Insights Blood Disord 2017;10:1179545X17699849.
- Ji Y, Chen S, Yang K, et al. Kaposiform hemangioendothelioma: current knowledge and future perspectives. Orphanet J Rare Dis 2020;15:39. [Crossref] [PubMed]
- Tower RL. Kaposiform haemangioendothelioma: new insights and old problems. Br J Dermatol 2018;179:253-4. [PubMed]
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr 2013;162:142-7. [Crossref] [PubMed]
- O'Rafferty C, O'Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol 2015;171:38-51. [Crossref] [PubMed]
- Schmid I, Klenk AK, Sparber-Sauer M, et al. Kaposiform hemangioendothelioma in children: a benign vascular tumor with multiple treatment options. World J Pediatr 2018;14:322-9. [Crossref] [PubMed]
- Liu XH, Li JY, Qu XH, et al. Treatment of kaposiform hemangioendothelioma and tufted angioma. Int J Cancer 2016;139:1658-66. [Crossref] [PubMed]
- Yao W, Li K, Wang Z, et al. Retroperitoneal kaposiform hemangioendothelioma complicated by Kasabach-Merritt phenomenon and obstructive jaundice: A retrospective series of 3 patients treated with sirolimus. Pediatr Dermatol 2020;37:677-80. [Crossref] [PubMed]
- Kim D, Yang HB, Kim HY. Malignant pancreatic tumor other than solid pseudopapillary tumor in pediatric patients: A single-center experience. Medicine (Baltimore) 2021;100:e27967. [Crossref] [PubMed]
- Mathew D, Mahomed N. Pancreatic kaposiform hemangioendothelioma complicated by Kasabach-Merritt phenomenon: A rare entity. SA J Radiol 2019;23:1760. [Crossref] [PubMed]
- Triana PJ, Dore M, Nuñez VC, et al. Pancreatic Kaposiform Hemangioendothelioma Not Responding to Sirolimus. European J Pediatr Surg Rep 2017;5:e32-5. [Crossref] [PubMed]
- Leung M, Chao NS, Tang PM, et al. Pancreatic kaposiform hemangioendothelioma presenting with duodenal obstruction and kasabach-merritt phenomenon: a neonate cured by whipple operation. European J Pediatr Surg Rep 2014;2:7-9. [Crossref] [PubMed]
- Wang C, Li Y, Xiang B, et al. Successful Management of Pancreatic Kaposiform Hemangioendothelioma With Sirolimus: Case Report and Literature Review. Pancreas 2017;46:e39-41. [Crossref] [PubMed]
- Ryu YJ, Choi YH, Cheon JE, et al. Imaging findings of Kaposiform Hemangioendothelioma in children. Eur J Radiol 2017;86:198-205. [Crossref] [PubMed]
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol 2004;28:559-68. [Crossref] [PubMed]
- Hall GW. Kasabach-Merritt syndrome: pathogenesis and management. Br J Haematol 2001;112:851-62. [Crossref] [PubMed]
- Freixo C, Ferreira V, Martins J, et al. Efficacy and safety of sirolimus in the treatment of vascular anomalies: A systematic review. J Vasc Surg 2020;71:318-27. [Crossref] [PubMed]
- Ji Y, Chen S, Xiang B, et al. Sirolimus for the treatment of progressive kaposiform hemangioendothelioma: A multicenter retrospective study. Int J Cancer 2017;141:848-55. [Crossref] [PubMed]
- Alvarado Y, Mita MM, Vemulapalli S, et al. Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Target Oncol 2011;6:69-94. [Crossref] [PubMed]
- Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer 2018;18:744-57. [Crossref] [PubMed]
- Kobayashi S, Kishimoto T, Kamata S, et al. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci 2007;98:726-33. [Crossref] [PubMed]
- Sun S, Chen S, Liu F, et al. Constitutive Activation of mTORC1 in Endothelial Cells Leads to the Development and Progression of Lymphangiosarcoma through VEGF Autocrine Signaling. Cancer Cell 2015;28:758-72. [Crossref] [PubMed]
- Wang Z, Yao W, Sun H, et al. Sirolimus therapy for kaposiform hemangioendothelioma with long-term follow-up. J Dermatol 2019;46:956-61. [Crossref] [PubMed]
- Ying H, Qiao C, Yang X, et al. A Case Report of 2 Sirolimus-Related Deaths Among Infants With Kaposiform Hemangioendotheliomas. Pediatrics 2018;141:S425-9. [Crossref] [PubMed]


