
Heterotopic squamous epithelial inclusion cyst in a cervical lymph node in a child: a case report and literature review
Introduction
Benign heterotopic epithelial inclusions in lymph nodes have been well reported, especially in the axillary region (1,2). Some scholars classify nodal epithelial inclusions into the following 3 main categories based on morphology: (I) those consisting only of glandular epithelium (which are the most common); (II) those consisting only of squamous epithelial inclusion cysts (which are the least common); and (III) those consisting of both glandular and squamous epithelium (2). The pathogenesis of this rare heterotopic phenomenon is unclear; however, theories for its pathogenesis include implantation/translocation, metaplasia, and/or embryonic rest (2,3).
In cervical lymph nodes, the common heterotopic epitheliums are thyroid follicular epitheliums (4) and salivary gland epitheliums (5); squamous epithelial inclusion cysts are rare. Indeed, only 3 cases have been reported in the literature, none of which have described their ultrasound (US) characteristics (6-8).
In this article, we report a squamous epithelial inclusion cyst in a cervical lymph node in a pediatric patient, and summarizes the patient’s medical history, progression, diagnosis, imaging (with an emphasis on ultrasonography), and laboratory findings, treatment methods, and follow-up outcomes. We also reviewed the three previously published literatures, expecting to discover some regularities in the occurrence, development, diagnosis, treatment and follow-up of the disease. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-255/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian/next of kin for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 6-year-old boy, who was in good health, presented with a non-tender mass on the right side of the neck 1 month earlier. There was no change in the mass after 7 days of oral anti-infective and external treatment. Some 7 days before admission, the mass gradually increased in size and became tender. The patient had no fever, no cough and expectoration, no vomiting, and no diarrhea. The patient had no history of surgery, no history of tuberculosis or contact history, and no family history of genetic diseases. The physical examination revealed a palpable 3.0 cm × 2.0 cm mass with tenderness and poor mobility in the right submandibular region. There was no redness and swelling to the superficial skin, no ulceration, and no vascular murmur was detected on auscultation. The laboratory tests showed an elevated C-reactive protein of 17 mg/L (normal range: 0–8 mg/L). Other laboratory tests, including the mycobacterium tuberculosis antibody test and T-cell test for tuberculosis infection, were negative. The chest X-ray was normal.
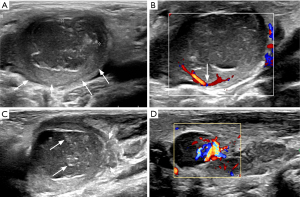
The US examination revealed an oval, ill-defined, 31 mm × 20 mm × 25 mm mass in the right submandibular region, consisting of a peripheral homogeneous hypoechoic component and an internal heterogeneous very hypoechoic component (see Figure 1). The child felt a little pain when the probe was pressurized.
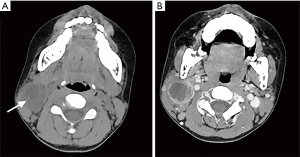
The computed tomography (CT) scan showed a heterogeneously hypodense mass with irregular annular enhancement in the right submandibular region (see Figure 2). The density of the fat space around the mass increased. The floor of the mouth, tongue, pharynx, and larynx were unremarkable. The remainder of the neck was normal.
Based on the patient’s medical history, physical examination, laboratory tests, ultrasonography and CT examinations, the preferred diagnosis was lymphadenopathy, but cervical neoplastic lesions could not be excluded. Thus, it was recommended that the patient undergo a coarse needle biopsy of the mass; however, the patient's family refused this operation, as it may cause bleeding or needle tract implantation transfer.
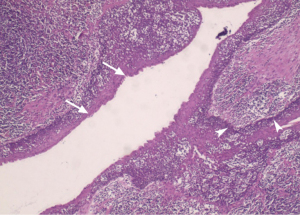
Surgery was performed to completely remove the mass. During the surgery, it was observed that the mass adhered to the surrounding tissues and was squeezing the ipsilateral external carotid artery. The pathology revealed that the squamous epithelial inclusion cyst replaced a considerable part of the node structure and was surrounded by normal lymphoid tissue and neutrophils (see Figure 3). Keratin debris was visible in the cyst. The pathology, along with the clinical findings, supported a final diagnosis of a secondary infection of squamous epithelial inclusion cysts in the right cervical lymph node.
At 1 week post-surgery, the swelling in the operation area had basically subsided, the wound had recovered well, and the blood routine and C-reactive protein tests were within the normal range. The parents of the child were satisfied with our treatment, and the patient was discharged from the hospital.
The patient was followed-up 3 times at 3 months, 6 months and 1 year after the surgery. The patient recovered well, and the physical examination revealed no swelling, pain, or dysfunction in the surgical area. Ultrasonography was performed at each follow-up, and showed that the surgical area recovered well, and no recurrence of the mass was found. The patient’s C-reactive protein also returned to normal. The parents of the child showed good compliance in the review, and we will continue to follow-up with the patient in the future. The timeline of the case was demonstrated in Figure 4.
Table 1 provides a summary of the 3 published cases of squamous epithelial inclusion cysts in a cervical lymph node. Of the 3 cases, 1 was male and 2 were female, and all were younger than 40 years old. In 2 of the 3 cases, as in our patient, the neck mass was located in the submandibular region; the other case did not mention the specific location of the mass in the neck. The 16-year-old female patient had a history of contralateral cervical lymph node tuberculosis 10 years ago, and was treated successfully, while the other 2 cases had no previous diagnoses of other diseases. All cases were treated with surgical resection, and no follow-up data were available.
Table 1
| References | Case | Age/sex | Location | Size, cm | Previous related neck disease |
|---|---|---|---|---|---|
| Fruehwald-Pallamar et al. (6) | 1 | 21/F | Submandibular region (L) | 1.7 | None |
| Bahadur et al. (7) | 2 | 16/F | Submandibular region (R) | 2.5×2.0 | Contralateral cervical lymph node tuberculosis 10 years ago, which was cured |
| Houcine et al. (8) | 3 | 38/M | Neck (NOS) | 2.5×2.0 | None |
F, female; M, male; NOS, not otherwise specified; R, right; L, left.
By reviewing the literature, we found that heterotopic squamous inclusion cysts in cervical lymph nodes are more common in young patients, and most of the lesions are located in the submandibular region.
Discussion
Heterotopic epithelial inclusions in lymph nodes have been identified in multiple anatomical sites; however, except for the axillary region, which has a high number of cases, all other anatomical regions have been reported as single cases (2,4,5,9,10). The most concerning aspect of benign epithelial inclusions in lymph nodes is that they may be misinterpreted as tumor metastases (1,11). Thus, the detailed morphological evaluation of lymph nodes is critical. The criteria for benign epithelial inclusions include bland nuclear cytology, no anaplastic lesions, and active mitosis (7). An additional concern is that benign inclusions in lymph nodes may also turn into malignant lesions. For example, benign epithelial inclusions in cervical lymph nodes have been shown to transform into papillary thyroid carcinoma (12), and benign epithelial inclusions in axillary lymph nodes have been shown to transform into ductal carcinoma in situ (13).
To date, no ultrasonographic descriptions of heterotopic squamous epithelial inclusion cysts in cervical lymph nodes have been provided in the literature, and there have only been a few reports of these cysts in other anatomical sites. Nakaguro et al. (9) reported an echogenic solid tumor, with well-defined edges, in the right breast. Kim et al. (10) reported a hypoechoic mass in the celiac region. These 2 cases were pathologically diagnosed as squamous epithelial inclusion cysts in the lymph nodes, but the gray-scale ultrasonographic findings were not specific, and no color Doppler features were described.
In our study, US revealed an oval, ill-defined mass in the right submandibular area, consisting of a peripheral homogeneous hypoechoic component with hilar-like vascularity and an internal heterogeneous very hypoechoic component with patchy hyperechoic areas. Except for the right neck mass, the other neck organs were normal on the US and CT examinations. These ultrasonographic features suggest that the mass is likely to originate from a lymph node abscess, which may be secondary to purulent lymphadenitis or tuberculous lymphadenitis. However, pathology confirmed that the internal heterogeneous hypoechoic component was mucus and keratin debris in the squamous inclusion cyst rather than abscesses, and the peripheral homogeneous hypoechoic component was normal lymph node tissue.
A wide variety of diseases can cause neck masses in children, including lymph node-related inflammation, congenital lesions, and neoplastic lesions. Some of these diseases may mimic squamous inclusion cysts in cervical lymph nodes. Thus, the disease must be differentiated from these other diseases. US is the first-line method of choice for evaluating neck masses in children. It provides rapid and economical information on the location, size, shape, internal contents, blood flow, and relationship of the mass to nearby vessels without the need for ionizing radiation and sedatives (14). The correlation of clinical and ultrasonographic findings is important to determine the most accurate preoperative diagnosis (15).
Cervical lymphadenitis is the most common cause of pediatric neck masses (14). As the condition of suppurative lymphadenitis progresses, abscesses may form inside the lymph nodes. US showed that the echoes in the lymph nodes were heterogeneous, often multilocular, and contained debris, septa, cavitation, or gas (bright echogenic foci with dirty shadowing); the blood flow signals around the nodule were increased, and the blood flow signals in the adjacent sternocleidomastoid muscle were also increased in severe cases (16). Additionally, the nodes may be fused together and appear as a single conglomerate mass (17). Clinically, most children will present with a high fever and leukocytosis in the blood. These features are different from the US appearance and clinical information of our case.
The early manifestations of tuberculous lymphadenitis are swollen, tangled, firm, non-tender lymph nodes with a S/L (short diameter/long diameter) ratio greater than 0.5. In the late stage of the disease, the manifestations are caseous degeneration, necrosis, thin echoes, strong echoes, and suppuration in the lymph nodes, which on the US appear as multiple irregularly shaped, heterogeneous, hypoechoic masses with enhanced posterior echogenicity, calcifications, with a capsular or peripheral vascular distribution (18). Eventually, the inflammation in the lymph nodes spreads into the surrounding soft tissue, forming an abscess (18).
Thyroglossal duct cyst (TDC) and branchial cleft anomalies are the top 2 congenital neck masses (19). TDC is most commonly found at the level of the hyoid bone (20). TDC also presents as a painful neck mass when secondary to infection, as in our cases. TDC on US presents as a cyst-like, thin-walled, anechoic or hypoechoic mass with clear borders, a regular shape, an enhanced posterior echo, and no blood flow; when there is septum, protein material or blood in the cyst, the US shows a very complex and hyperechoic internal echo; when the cyst becomes infected, the cyst wall becomes thick and irregular, and the cyst wall may show color Doppler flow signals (14,16). These ultrasonographic features of TDC are similar to those of our cases. However, 75% of the TDC is in the midline of the neck and 25% is slightly off the midline (it is always within 2 cm of the midline) (16), and it shows a spontaneous rise with swallowing and sticking the tongue out (21), neither of which was present in our case.
Branchial cleft anomalies are common in children, including branchial cleft cyst (BCC), sinus tracts, or fistulae. Second BCCs are the most common branchial cleft anomalies and require imaging evaluation (22). BCCs are mostly located in the mandibular space, and thus have the same location as our cases. On ultrasonography, a BCC appears as an oval anechoic mass with thin walls, well-defined borders, and no blood flow (16). When the cyst is infected, the clinical manifestation is a painful mass in the neck, and the US shows an irregular thickening of the cyst wall and internal echo enhancement. Thus, BCC needs to be carefully differentiated from our case.
Neck malignant tumors in children are rare. Lymphomas, which account for 50% of such tumors, are the most common pediatric head and neck malignancy, followed by rhabdomyosarcomas, which account for 10–15% of such tumors (16). US of lymphoma shows enlarged, round, hypoechoic nodes, absent hilum, and internal reticulation; a color Doppler US shows increased central and peripheral blood flow; a spectral Doppler US shows an increased resistance index and pulsatile index; the nodes can become confluent, matted, and mass-like (14). Rhabdomyosarcoma is low-to-moderately echogenic on gray-scale ultrasonography with variable internal vascularity on color Doppler ultrasonography (16). The sonographic appearance of cervical malignancies is significantly different from our case.
In conclusion, heterotopic squamous inclusion cysts in cervical lymph nodes have typical ultrasonographic features. US combined with basically normal laboratory test results (normal blood routine examination and negative tuberculosis-related laboratory test results), can provide an early indication for this rare disease. At the same time, we reviewed the literature and found that this heterotopic phenomenon was more likely to occur in the submandibular region of the neck in younger patients. For children, if Doppler ultrasonography shows an oval, ill-defined mass in the submandibular area, consisting of a peripheral homogeneous hypoechoic component with hilar-like vascularity and an internal heterogeneous very hypoechoic component with patchy hyperechoic areas, in addition to a diagnosis of suppurative lymphadenitis, tuberculous lymphadenitis, or an infected second branchial cleft cyst, the possibility of a squamous epithelial inclusion cyst in the lymph node should be considered.
There is no standardized treatment for this rare disease. Surgical resection was used in all 3 cases in the literature and in our case. Surgery may be an effective treatment for this disease, but more cases are still needed to prove it.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-255/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-255/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rutty GN. Benign lymph node inclusions. J Pathol 1994;173:301-2. [Crossref] [PubMed]
- Fellegara G, Carcangiu ML, Rosai J. Benign epithelial inclusions in axillary lymph nodes: report of 18 cases and review of the literature. Am J Surg Pathol 2011;35:1123-33. [Crossref] [PubMed]
- Moreno G, Jorns JM. Endosalpingiosis and other benign epithelial inclusions in breast sentinel lymph nodes. Breast J 2020;26:274-5. [Crossref] [PubMed]
- León X, Sancho FJ, García J, et al. Incidence and significance of clinically unsuspected thyroid tissue in lymph nodes found during neck dissection in head and neck carcinoma patients. Laryngoscope 2005;115:470-4. [Crossref] [PubMed]
- Daniel E, McGuirt WF Sr. Neck masses secondary to heterotopic salivary gland tissue: a 25-year experience. Am J Otolaryngol 2005;26:96-100. [Crossref] [PubMed]
- Fruehwald-Pallamar J, Li CQ, Hasteh F, et al. Nodal Inclusion Cyst in a Cervical Lymph Node. Neurographics 2012;2:163-6. [Crossref]
- Bahadur S, Pujani M, Jetley S. Epithelial Inclusion Cyst in a Cervical Lymph Node: Report of a Rare Entity at an Uncommon Location. Ann Med Health Sci Res 2016;6:137-8. [Crossref] [PubMed]
- Houcine Y, Sassi A, Mlika M, et al. A rare case of squamous inclusion cyst in cervical lymph node. Heliyon 2020;6:e04225. [Crossref] [PubMed]
- Nakaguro M, Suzuki Y, Ichihara S, et al. Epithelial inclusion cyst arising in an intramammary lymph node: case report with cytologic findings. Diagn Cytopathol 2009;37:199-202. [Crossref] [PubMed]
- Kim SB, Kim KH, Kim TN, et al. A rare case of an enlarged celiac lymph node diagnosed as an epidermal inclusion cyst. Korean J Intern Med 2020;35:480-1. [Crossref] [PubMed]
- Caneve P, Fraune C. Benign Müllerian-type epithelial inclusions in a lymph node of a patient with prostatic adenocarcinoma: Avoiding overdiagnosis of metastases. Pathologe 2022;43:140-2. [Crossref] [PubMed]
- Wang Z, Qiu S, Eltorky MA, et al. Histopathologic and immunohistochemical characterization of a primary papillary thyroid carcinoma in the lateral cervical lymph node. Exp Mol Pathol 2007;82:91-4. [Crossref] [PubMed]
- Srinivasan B, Allan CP, Armes JE. Ductal carcinoma in situ arising in an epithelial inclusion within an axillary lymph node. Pathology 2007;39:268-9. [Crossref] [PubMed]
- Bansal AG, Oudsema R, Masseaux JA, et al. US of Pediatric Superficial Masses of the Head and Neck. Radiographics 2018;38:1239-63. [Crossref] [PubMed]
- Rankovic N, Todorovic J, Simic R. Clinical and ultrasound characteristics of pediatric lateral neck masses. PLoS One 2021;16:e0251563. [Crossref] [PubMed]
- Rosenberg HK. Sonography of pediatric neck masses. Ultrasound Q 2009;25:111-27. [Crossref] [PubMed]
- van den Brekel MW, Castelijns JA, Snow GB. Imaging of cervical lymphadenopathy. Neuroimaging Clin N Am 1996;6:417-34. [PubMed]
- Yu TZ, Zhang Y, Zhang WZ, et al. Role of ultrasound in the diagnosis of cervical tuberculous lymphadenitis in children. World J Pediatr 2021;17:544-50. [Crossref] [PubMed]
- Pupić-Bakrač J, Pupić-Bakrač A, Novaković J, et al. Congenital Neck Masses. J Craniofac Surg 2021;32:1417-20. [Crossref] [PubMed]
- Bezerra Júnior GL, Silva LF, Pimentel GG, et al. Treatment of Large Thyroglossal Duct Cyst. J Craniofac Surg 2017;28:e794-5. [Crossref] [PubMed]
- Arredondo Montero J, Bronte Anaut M, Antona G, et al. Thyroglossal Duct Cyst: Ascension with Swallowing. J Pediatr 2021;238:330-1. [Crossref] [PubMed]
- Pupić-Bakrač J, Skitarelić N, Pupić-Bakrač A, et al. Branchial cleft anomalies: hybrid "Branchial Inclusion" theory. Eur Arch Otorhinolaryngol 2021;278:2593-601. [Crossref] [PubMed]



