
Efficacy and safety of nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure ventilation in neonatal respiratory distress syndrome: a systematic review and meta-analysis
Introduction
Respiratory distress syndrome (RDS) is the most common respiratory disease in newborns, especially premature infants (1-3). About 40% of RDS newborns require mechanical ventilation (4,5), which is a risk factor for adverse complications such as bronchopulmonary dysplasia (BPD) and atelectasis (6-8). Reducing endotracheal intubation and mechanical ventilation is an important goal in the clinical treatment of neonatal RDS (9). As a non-invasive mode of ventilation, nasal continuous positive airway pressure (NCPAP) provides early respiratory support for RDS newborns (10-13). However, the therapeutic effect of NCPAP is not ideal, and about half of patients experience treatment failure.
Nasal intermittent positive pressure ventilation (NIPPV) is an essential non-invasive respiratory support model; it has been applied in clinics and has gradually become more popular. However, the specific mechanism of NIPPV has not been fully clarified. The increased pressure of NIPPV is transmitted to the lower respiratory tract. Increasing tidal volume and minute ventilation may be the primary mechanism of NIPPV in reducing endotracheal intubation (14). The increased pressure of NIPPV may be used as a stimulus to reduce the onset of apnea, increase mean airway pressure, increase lung volume, and support alveolar dilatation. NIPPV can also reduce physiological dead space and increase gas exchange by promoting the elimination of exhaled gas (14).
The efficacy of NIPPV and NCPAP are two different non-invasive ventilation modes. In terms of safety, they have considerable advantages, but there are controversies in the treatment efficacy of neonatal RDS. Previous studies (15,16) have shown that NIPPV can reduce the incidence of endotracheal intubation and BPD. No adverse events were observed in NIPPV and NCPAP. However, a recent randomized controlled trial (RCT) (17) reported no difference in the incidence of endotracheal intubation between NIPPV and NCPAP in the treatment of neonatal RDS. NIPPV does not provide benefits to RDS newborns in terms of mortality, BPD, sepsis, and atelectasis. We believed that the reasons for these differences in results are the selection bias of research objects and the small sample size. A previous meta-analysis (18) and its results update (19) showed that NIPPV could reduce the incidence of endotracheal intubation. However, this effect is conditional and cannot be promoted. Also, this meta-analysis (18) and results update (19) included fewer studies, and the results were limited to the benefits of NIPPV in reducing the demand for invasive ventilation. Therefore, we believe that a meta-analysis is necessary to compare the efficacy and safety of NIPPV and NCPAP in neonatal RDS. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-288/rc).
Methods
Literature retrieval
The literature search was conducted based on the literature retrieval was performed in PubMed, EMBASE, Medline, Central, China National Knowledge Infrastructure (CNKI), Wanfang and China Science Digital Library (CSDL) databases. The search terms were as follows: (“nasal intermittent positive pressure ventilation” OR “NIPPV”) AND (“nasal continuous positive airway pressure” OR “NCPAP”) AND (“respiratory distress syndrome” OR “neonatal respiratory distress syndrome” OR “RDS”). The search deadline was May 17, 2022, and there were no language restrictions.
Literature screening
Inclusion criteria: (I) subjects: newborns with RDS; (II) studies that had established both experimental and control groups; (III) the intervention measures of the experimental and control groups were NIPPV and NCPAP, respectively; (IV) the results included the incidence of intubation, BPD, or mortality; and (V) RCTs.
Exclusion criteria: (I) repeated reports and case reports; (II) studies with a poor balance of baseline data between the experimental and control groups; (III) other non-invasive ventilation modes were applied in the experimental or control groups; (IV) literatures in which key data were missing and could not be supplemented.
Data extraction
Two researchers extracted the data including the author, title, publication time, number of research cases, intubation needs, number of deaths, number of BPD cases, etc. The literature author was contacted in cases where the data missing. The two researchers resolved their differences of opinion through discussion.
Literature quality evaluation
In this paper, two researchers used the revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB2.0) to evaluate the quality of the included RCT research. RoB consists of five items, namely randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The literature was divided into three categories: low risk of bias, high risk of bias, and uncertain. As long as there was a high risk of bias in one of all items, the literature had a high risk of bias. If all items had low risk of bias, the literature had low risk of bias. In addition to the above two cases, the risk of literature bias was uncertain.
Statistical method
The Cochrane software (RevMan5.3) was applied to analyze the data. The risk ratio (RR) and 95% confidence interval (CI) were used to describe the effect quantity. The Chi-square test was applied for heterogeneity assessment. When the I2>50% or P<0.1, there was heterogeneity among the included literature, and a random effects model was conducted. The causes of heterogeneity were explored via subgroup analysis. However, when the I2≤50% and P≥0.1, no heterogeneity existed among the published literature, and the fixed effects model was conducted. The funnel plot and egger test were used to test for publication bias. Sensitivity analysis was used to assess the effect of literature bias risk on the stability of results. Bilateral P<0.05 was considered to indicate statistical significance.
Results
Characteristics of included literature
We retrieved 1,867 literatures from the database, and finally selected 10 literatures for study (15-17,20-26). The 10 literatures included 1,104 patients, 557 in the NIPPV group and 547 in the NCPAP group. The screening flow chart is shown in Figure 1. The characteristic information and risk of bias of the literatures are shown in Table 1. Among the literatures included in our analysis, 2 literatures had low risk of bias, 2 literatures had high risk of bias, and the rest had uncertain risk of bias (Table 2).
Table 1
| Author | Year | Study type | No. of patients | Risk of bias | |
|---|---|---|---|---|---|
| NIPPV | NCPAP | ||||
| Bhandari (20) | 2007 | RCT | 20 | 21 | Low risk |
| Estay (17) | 2020 | RCT | 112 | 108 | Uncertain |
| Jasani (21) | 2016 | RCT | 31 | 32 | Uncertain |
| Kahramaner (22) | 2014 | RCT | 39 | 28 | Uncertain |
| Khalaf (23) | 2001 | RCT | 34 | 30 | High risk |
| Sai Sunil Kishore (24) | 2009 | RCT | 37 | 39 | Uncertain |
| Kugelman (15) | 2007 | RCT | 43 | 41 | Low risk |
| Oncel (16) | 2016 | RCT | 100 | 100 | Uncertain |
| Ramanathan (25) | 2012 | RCT | 53 | 57 | High risk |
| Shi (26) | 2014 | RCT | 88 | 91 | Uncertain |
NIPPV, nasal intermittent positive pressure ventilation; NCPAP, nasal continuous positive airway pressure; RCT, randomized controlled trial.
Table 2
| Author | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall |
|---|---|---|---|---|---|---|
| Bhandari (20) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Estay (17) | Uncertain | Low risk | Low risk | Low risk | Low risk | Uncertain |
| Jasani (21) | Low risk | Uncertain | Low risk | Low risk | Uncertain | Uncertain |
| Kahramaner (22) | Low risk | Low risk | Low risk | Uncertain | Low risk | Uncertain |
| Khalaf (23) | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Sai Sunil Kishore (24) | Low risk | Uncertain | Low risk | Low risk | Low risk | Uncertain |
| Kugelman (15) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Oncel (16) | Low risk | Uncertain | Low risk | Uncertain | Low risk | Uncertain |
| Ramanathan (25) | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Shi (26) | Low risk | Low risk | Low risk | Uncertain | Low risk | Uncertain |
Comparison of intubation rates between the NIPPV and NCPAP groups
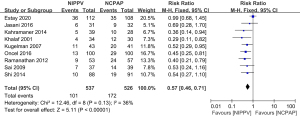
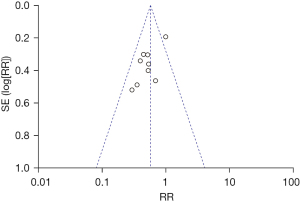
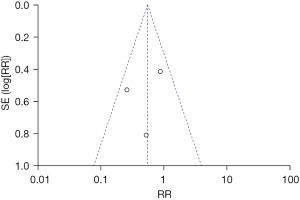
A total of nine literatures were about to the incidence of intubation between the NIPPV and NCPAP groups. There were 537 RDS newborns in the NIPPV group, 101 of whom required intubation, and 526 RDS newborns in the NCPAP group, 172 of whom needed intubation. The heterogeneity test showed that there was no heterogeneity among the studies (Chi2=12.46, P=0.13, I2=36%). NIPPV reduced the incidence of neonatal intubation in RDS newborns compared with NCPAP (RR =0.57, 95% CI: 0.46–0.71, Z=5.11, P<0.00001, Figure 2). The funnel plot and egger test showed that the scatter points were biased to the left with publication bias (P<0.05, Figure 3).
Comparison of BPD incidence between the NIPPV and NCPAP groups
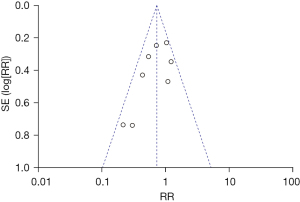
A total of eight literatures were included to compare the incidence of BPD between the NIPPV and NCPAP groups. In the NIPPV group, there were 484 RDS newborns and 95 had BPD. There were 470 RDS newborns in the NCPAP group and 125 had BPD. The heterogeneity test showed that there was no heterogeneity among the studies (Chi2=11.93, P=0.10, I2=41%). NIPPV reduced the incidence of BPD in RDS newborns compared with NCPAP (RR =0.72, 95% CI: 0.57–0.91, Z=2.70, P=0.007, Figure 4). The funnel plot and egger test indicated the scatter points were distributed roughly symmetrical, and there was no publication bias (P>0.05, Figure 5).

Comparison of mortality between the NIPPV and NCPAP groups
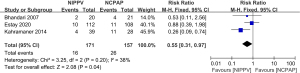
Three literatures were included to compare the mortality between the NIPPV and NCPAP groups. There were 171 RDS newborns in the NIPPV group and 15 died. There were 157 RDS newborns in the NCPAP group and 26 died. The heterogeneity test showed that there was heterogeneity among the studies (Chi2=3.25, P=0.20, I2=38%). NIPPV reduced the neonatal mortality rate of RDS compared with NCPAP (RR =0.55, 95% CI: 0.31–0.97, Z=2.08, P=0.04, Figure 6). The funnel plot and egger test indicated the scatter points were distributed roughly symmetrical, and there was no publication bias (P>0.05, Figure 7).
Discussion
Our meta-analysis showed that NIPPV could reduce the incidence of neonatal intubation, BPD, and mortality compared with NCPAP. Some previous studies have suggested that NIPPV can benefit RDS newborns. Bhandari et al. (20) compared the effects of NIPPV and NCPAP on the prognosis of newborns with RDS and found that NIPPV could significantly reduce the incidence of neonatal BPD and death. NIPPV and NCPAP were similar in preventing other diseases or complications. Also, the two treatments had similar effects on neonatal neurodevelopment. Kahramaner et al. (22) found that compared with NIPPV, NCPAP might increase the incidence of atelectasis and intubation in premature infants with RDS after treatment. However, no difference was observed in the incidence of BPD, sepsis, and pneumothorax between NIPPV and NCPAP, but children treated with NIPPV had a lower mortality rate. Khalaf et al. (23) demonstrated that NIPPV significantly reduced the incidence of reintubation in RDS newborns after treatment, as compared with NCPAP. Moreover, the benefits offered by NIPPV may be more significant if the child’s weight is controlled. There was no difference in apnea or bradycardia between the two groups.
Also, children with NIPPV had better pulmonary dynamic compliance. Sai Sunil Kishore et al. (24) suggested that NIPPV could reduce the proportion of intubation and mechanical ventilation in RDS newborns. The efficacy of NIPPV was superior to NCPAP in newborns at 28–30 and 31–34 weeks, which was independent of the use of surfactants. Sai Sunil Kishore et al. (24) suggested that premature infants with a high-RDS risk could be treated with NIPPV. Kugelman et al. (15) showed that the incidence of endotracheal intubation in RDS preterm infants treated with NIPPV was lower than that in those treated with NCPAP. The efficacy advantage of NIPPV is more significant in preterm infants with low body weight and small for gestational age.
NIPPV significantly reduced the incidence of BPD in premature infants with RDS. This trend still exists in preterm infants weighing less than 1500g. In their study of 200 premature infants with RDS, Oncel et al. (16) showed that NIPPV could reduce the proportions of RDS premature infants requiring surfactant treatment as well as the use of surfactant (16). The incidence of intubation and BPD in children treated with NIPPV was lower than in those treated with NCPAP. Oncel et al. (16) suggested that premature infants with a gestational age of 26–32 weeks should be treated with NIPPV instead of NCPAP after RDS. Ramanathan et al. (25) showed that NIPPV was more effective than NCPAP in preterm infants <30 weeks. Furthermore, NIPPV could reduce the proportion of children’s need for invasive ventilation and the treatment time of invasive ventilation. NIPPV also reduced the incidence of pathological BPD.
Shi et al. (26) compared the efficacy of NIPPV and NCPAP on RDS in both preterm and term infants. NIPPV reduced the incidence of intubation and the need for mechanical ventilation. This result was observed in both preterm and term infants. The food intake and body weight of the children in the NIPPV group were higher than those in the NCPAP group. Thus, NIPPV can improve the prognosis of children with RDS.
The above research results support that NIPPV can benefit RDS newborns by reducing invasive ventilation. However, different views have been proposed in some literatures. Jasani et al. (21) showed NCPAP and NIPPV had similar incidence of intubation in premature infants with RDS. NIPPV also has advantages in reducing the oxygen inhalation time and the incidence of BPD. Another recent RCT (17) showed that NIPPV and NCPAP had similar effects on neonatal RDS; there was no reported difference in the incidence of intubation, BPD, and mortality between the NIPPV and NCPAP groups. Moreover, there was also no difference in the incidence of intraventricular hemorrhage, pneumothorax, and invasive ventilation time between the two groups.
A previous meta-analysis showed that (18) NIPPV could reduce the need for invasive ventilation in preterm infants but could not reduce the incidence of BPD. However, only three studies were included in this meta-analysis, and the number of cases was small. Li et al. (19) updated the results of this meta-analysis; they believe that the efficacy of NIPPV is limited and may be related to the weight and gestational age of premature infants. However, their study was limited to the benefit of NIPPV in terms of the incidence of intubation. We suggest that compared with NCPAP, NIPPV can provide benefits in terms of the incidence of intubation, BPD, and mortality.
There were some defects in our study. Firstly, there were not enough included literatures and small sample size; secondly, some included literatures had high risk of bias. These may have had an impact on the results.
In conclusion, compared with NCPAP, NIPPV can reduce the incidence of intubation, BPD, and mortality of NCPAP. The conclusions need to be confirmed via high-quality RCTs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-288/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-288/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Montan S, Arulkumaran S. Neonatal respiratory distress syndrome. Lancet 2006;367:1878-9. [Crossref] [PubMed]
- Raimondi F, Migliaro F, Corsini I, et al. Lung Ultrasound Score Progress in Neonatal Respiratory Distress Syndrome. Pediatrics 2021;147:e2020030528. [Crossref] [PubMed]
- Reynolds P, Bustani P, Darby C, et al. Less-Invasive Surfactant Administration for Neonatal Respiratory Distress Syndrome: A Consensus Guideline. Neonatology 2021;118:586-92. [Crossref] [PubMed]
- Stylianou-Riga P, Boutsikou T, Kouis P, et al. Maternal and neonatal risk factors for neonatal respiratory distress syndrome in term neonates in Cyprus: a prospective case-control study. Ital J Pediatr 2021;47:129. [Crossref] [PubMed]
- Zou J, Gu L. Effects of comprehensive care on complications, oxygenation indexes and guardian's psychological mood of children with neonatal respiratory distress syndrome. Am J Transl Res 2021;13:5147-55. [PubMed]
- Chen D, Liu X, Li J. Mechanical Ventilation in Neonatal Respiratory Distress Syndrome at High Altitude: A Retrospective Study From Tibet. Front Pediatr 2019;7:476. [Crossref] [PubMed]
- Liang Z, Meng Q, You C, et al. Roles of Lung Ultrasound Score in the Extubation Failure From Mechanical Ventilation Among Premature Infants With Neonatal Respiratory Distress Syndrome. Front Pediatr 2021;9:709160. [Crossref] [PubMed]
- Wang LP, Mao QH, Yang L. Effect of pulmonary surfactant combined with mechanical ventilation on oxygenation functions and expressions of serum transforming growth factor-beta1 (TGF-β1) and bone morphogenetic protein 7 (BMP-7) of neonatal respiratory distress syndrome. Eur Rev Med Pharmacol Sci 2017;21:4357-61. [PubMed]
- Huo MY, Mei H, Zhang YH, et al. Efficacy and safety of less invasive surfactant administration in the treatment of neonatal respiratory distress syndrome: a Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:721-7. [PubMed]
- Gao Y, Chen X, Zhang Z, et al. Efficacy and safety of inhalation of pulmonary surfactant using vibrating mesh nebulizers combined with nasal continuous positive airway pressure in the treatment of neonatal respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2022;34:80-4. [PubMed]
- Shu XX, Chen C, Tang J, et al. Clinical effect of bubble nasal continuous positive airway pressure versus conventional nasal continuous positive airway pressure in respiratory support for preterm infants with neonatal respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi 2018;20:433-7. [PubMed]
- Wang JJ, Zhang L, Cai N. A comparative study of the efficacy and safety of high-flow nasal cannula and nasal continuous positive airway pressure in neonatal respiratory distress syndrome: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e29109. [Crossref] [PubMed]
- Zhang C, Zhu X. Clinical effects of pulmonary surfactant in combination with nasal continuous positive airway pressure therapy on neonatal respiratory distress syndrome. Pak J Med Sci 2017;33:621-5. [Crossref] [PubMed]
- Rüegger CM, Owen LS, Davis PG. Nasal Intermittent Positive Pressure Ventilation for Neonatal Respiratory Distress Syndrome. Clin Perinatol 2021;48:725-44. [Crossref] [PubMed]
- Kugelman A, Feferkorn I, Riskin A, et al. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized, controlled, prospective study. J Pediatr 2007;150:521-6, 526.e1.
- Oncel MY, Arayici S, Uras N, et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2016;101:F323-8. [Crossref] [PubMed]
- Estay AS, Mariani GL, Alvarez CA, et al. Randomized Controlled Trial of Nonsynchronized Nasal Intermittent Positive Pressure Ventilation versus Nasal CPAP after Extubation of VLBW Infants. Neonatology 2020;117:193-9. [Crossref] [PubMed]
- Meneses J, Bhandari V, Alves JG. Nasal intermittent positive-pressure ventilation vs nasal continuous positive airway pressure for preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Pediatr Adolesc Med 2012;166:372-6. [Crossref] [PubMed]
- Li W, Long C, Zhangxue H, et al. Nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure for preterm infants with respiratory distress syndrome: a meta-analysis and up-date. Pediatr Pulmonol 2015;50:402-9. [Crossref] [PubMed]
- Bhandari V, Gavino RG, Nedrelow JH, et al. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. J Perinatol 2007;27:697-703. [Crossref] [PubMed]
- Jasani B, Nanavati R, Kabra N, et al. Comparison of non-synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as post-extubation respiratory support in preterm infants with respiratory distress syndrome: a randomized controlled trial. J Matern Fetal Neonatal Med 2016;29:1546-51. [Crossref] [PubMed]
- Kahramaner Z, Erdemir A, Turkoglu E, et al. Unsynchronized nasal intermittent positive pressure versus nasal continuous positive airway pressure in preterm infants after extubation. J Matern Fetal Neonatal Med 2014;27:926-9. [Crossref] [PubMed]
- Khalaf MN, Brodsky N, Hurley J, et al. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics 2001;108:13-7. [Crossref] [PubMed]
- Sai Sunil Kishore M, Dutta S, Kumar P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatr 2009;98:1412-5. [Crossref] [PubMed]
- Ramanathan R, Sekar KC, Rasmussen M, et al. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants <30 weeks' gestation: a randomized, controlled trial. J Perinatol 2012;32:336-43. [Crossref] [PubMed]
- Shi Y, Tang S, Zhao J, et al. A prospective, randomized, controlled study of NIPPV versus nCPAP in preterm and term infants with respiratory distress syndrome. Pediatr Pulmonol 2014;49:673-8. [Crossref] [PubMed]



