
Surgical removal of bilateral lung metastases from Wilms tumor via subxiphoid approach video-assisted thoracic surgery: a case report
Introduction
According to the American Cancer Society’s 2021 cancer statistics report, patients with Wilms tumor (WT) and other nonepithelial renal tumors accounted for 5% of all children with malignant tumors (1). Among these patients, the lung is the most common site of metastasis (2). For patients without complete response of lung metastases after chemotherapy, the 5-year event-free survival (EFS) rate and overall survival (OS) rate are significantly reduced (3). Thus, surgical resection of lung metastases has become the most important means to address this situation. For patients with bilateral lung metastases, lateral chest approach video-assisted thoracic surgery (LCA-VATS) has been the predominant universal option until now. Although subxiphoid approach video-assisted thoracic surgery (SA-VATS) has become increasingly mature in adult thoracic surgery, there is no precedent for its application in pediatric thoracic surgery. We hereby report a case of a pediatric patient with bilateral lung metastases from WT treated via SA-VATS. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-102/rc).
Case presentation
Case
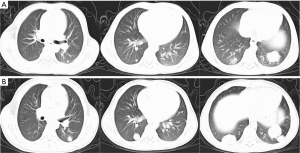
The parents of an 8-year-old child inadvertently discovered that he had abdominal enlargement on 10 August 2020. An initial color ultrasound showed a hypoechoic mass occupying the upper pole of the left kidney. The volume of the mass was approximately 12.4 cm × 9.9 cm × 10.8 cm. Enhanced computerized tomography (CT) of the chest and abdomen was highly indicative of left nephroblastoma with lung metastasis. The craniocerebral CT and emission computed tomography (ECT) showed no signs of distant metastasis in the brain or bones. The renal tumor was confirmed as nephroblastoma by ultrasound-guided percutaneous renal biopsy on 21 August 2020. The patient immediately underwent chemotherapy containing anthracycline for 6 weeks, from 25 August to 9 September 2020, according to the Chinese WT-2019 (2) protocol. A chest CT on 5 October 2020, showed multiple nodules in the inferior lobes of both sides of the lung (Figure 1A). On 9 October 2020, the patient successfully underwent abdominal surgery, which included left nephrectomy and dissection of the perinephric lymph nodes. Postoperative pathology confirmed that the viable tumor was a nephroblastoma composed of epithelium (66%), blastema (14%), and stroma (20%). Necrotic tumor tissue accounted for about 22% of the tumor. No tumor tissue was found in the perirenal fat, ureters, and renal hilum lymph nodes (0/11). From 19 October 2020 to 26 March 2021, the patient underwent postoperative chemotherapy containing cyclophosphamide, etoposide, vincristine, actinomycin-D, carboplatin, ifosfamide, and doxorubicin, according to the M protocol; however, a CT on 12 March 2021, showed that all of the lung metastases were larger than before (Figure 1B). Since diagnosis, the patient had not experienced any respiratory symptoms, such as shortness of breath or cough. The physical examination showed no obvious abnormalities. Since the patient had not achieved complete resolution (CR) of the lung metastases after postoperative chemotherapy, lung metastases resection was deemed necessary to ensure long-term survival. We decided to perform one-stage SA-VATS to preserve aesthetic appearance and ensure complete resection of all metastases in the inferior lobes of both lungs.
Surgical technique
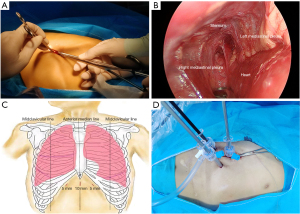
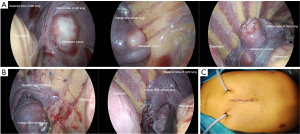
After general anesthesia, the patient was placed in the supine position. The patient’s back was bilaterally elevated to the level of the nipple line. First, a 1 cm longitudinal subxiphoid incision was made on the anterior midline. The Allis clamp was used to lift the xiphoid process upward. Sponge forceps and a thoracoscope were placed against the posterior wall of the sternum through this incision (Figure 2A). Dissociating the fiber and connective tissues of the anterior mediastinum by sponge forceps until the mediastinal pleura were seen on both sides (Figure 2B). Then, 5-mm incisions were made at the intersection of the lower margin of the costal arch and the 1 cm lateral to the parasternal line on both sides (Figure 2C). Then 35-mm trocars were respectively inserted through the above incisions (Figure 2D). First, the left mediastinal pleura was opened. A pneumoperitoneum machine was used to establish an artificial pneumothorax. The pressure was maintained at 7 mmHg with a flow rate of 4 L/min. After the left lung metastases were processed under right lung ventilation, we opened the right mediastinal pleura for resection of the right lung metastases under left lung ventilation. All pulmonary metastases were exophytic (Figure 3A). All surfaces of pulmonary metastases were white and had clear borders with the surrounding pulmonary tissue. The electrocoagulation hook was used to cauterize or mark the surface of lung tissue approximately 1 cm away from the tumor border. Then we used LigaSure (Medtronic, Minneapolis, MN, USA) to perform irregular resection of the lung tissue and metastases along the marked points. The lung resection margins were then sutured (Figure 3B). The pleural cavities on both sides were rinsed with warm distilled water, and no air leakage was detected at the lung incisal margins at 25 mmHg pressure. The patient’s intraoperative blood loss was approximately 20 mL. Drainage tubes were indwelled in both pleural cavities (Figure 3C).
Postoperative management
After surgery, the patient recovered from anesthesia smoothly, and the endotracheal intubation was successfully removed 40 minutes after surgery. No complications such as hypoxemia and respiratory failure occurred after surgery. After surgery, the patient was sent back to the observation room of the general ward for electrocardiogram and blood oxygen saturation monitoring. The patient was given low-flow nasal catheter oxygen at a flow rate of 2 L/min. Prophylactic antibiotics and analgesics were administered. On post-operative day (POD) 1, the patient left the observation room without oxygen and machine monitoring. The left and right thoracic drainage tubes were removed on POD 5 and POD 6, respectively. The X-ray taken before discharge indicated good recovery of pulmonary ventilation without pleural effusion or pneumothorax. The patient was discharged on POD 7.
Pathological results
The surgical specimens were 2 pieces of lung tissue. A total of 5 complete tumor bodies were found in the lung tissue. The transected surfaces of the tumors were gray-white in color, each with a tough texture exhibiting an exophytic growth pattern. The largest tumor was approximately 3.5 cm × 4 cm. Metastatic nephroblastoma tissue was observed in the tumor. Immunohistochemistry showed WT-1 (+), PAX-2 (+), PAX-8 (+), EMA (+), and Ki-67 (60%+). Taking the patient’s history into account, a pathological diagnosis was made of multiple metastases of nephroblastoma in both lungs after chemotherapy.
Postoperative follow-up
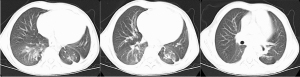
The patient had no obvious symptoms such as shortness of breath, cough, and chest pain within 4 months after discharge. A chest CT on 2 August 2021 showed good ventilation in both lungs and no obvious signs of tumor recurrence (Figure 4). The patient is currently receiving ongoing chemotherapy according to the established protocol.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was signed by the patients’ parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Among pediatric WT patients, 10% have lung metastases (2), which are usually confirmed as bilateral through preoperative CT. The Society of Pediatric Oncology (SIOP) 93-01 study and the TW2003 study conducted by the Italian Association of Pediatric Hematology and Oncology (AIEOP) in 2017 delineated the importance of chemotherapy for such patients (3). However, some lung metastases are not sensitive to chemotherapy. If lung metastases do not attain CR after initial chemotherapy, surgical removal becomes the only treatment option. In 2012, a study conducted by Verschuur et al. on 234 patients with nephroblastoma complicated by lung metastasis showed that the 5-year EFS rate of patients with resection of lung metastases after chemotherapy was 92%, which was superior to that of patients achieving CR after chemotherapy alone (5-year EFS rate: 88%) and those with multiple unresectable lung metastases (5-year EFS rate: 48%) (4). Therefore, a surgical operation via SA-VATS combined with timely postoperative chemotherapy was the best therapy for this patient.
There is no precedent in pediatric thoracic surgery for one-stage bilateral lung wedge resection via SA-VATS. At present, there is a unified guideline (UMBRELLA protocol SIOP 2014) for European surgeons for the selection of surgical methods or the lung resection range for these patients. The protocol recommend that bilateral resectable lung metastases should be excised either via two thoracotomies or one sternotomy depending on surgical choice and anatomy. Wedge resections can frequently be radical for therapy. If wedge resection will not achieve complete resected then segmentectomy or lobectomy is acceptable (5). But there is no unified international guideline for these patients. Another prevailing view indicate that children with bilateral lung metastases should accept 2 separate surgeries with an interval of 1–4 weeks (6). Thus, the scheduling of postoperative chemotherapy is further delayed in two-stage surgery compared with that following surgery for unilateral pulmonary metastasis. In the case presented in this paper, the ranges of the metastatic tumors in the lower lobe of both lungs were relatively limited. None of the tumors were close to the hilus pulmonis. Therefore, the choice of wedge resection of both lungs via one-stage SA-VATS was reasonable. This not only shortened the interval between the operation and other postoperative treatment, but also maximally preserved the lung function of the child.
Thoracotomy can cause significant additional damage to the chest walls of children. Some children, therefore, experience many postoperative complications, such as winged scapula (7). Due to the higher requirements regarding the aesthetics of chest shape and skin in pediatric patients compared to adults, LCA-VATS has frequently proven a better option than thoracotomy. Although LCA-VATS reduces the incidence of chest wall deformity, such as scapula winging, postoperative complications such as ipsilateral shoulder elevation and nipple asymmetry still exist, according to research by Lam et al. (8). The LCA-VATS approach has caused intercostal nerve injury to varying degrees (9), and research has shown that the incidence of post-thoracotomy pain syndrome (PTPS) following SA-VATS is lower than that experienced following LCA-VATS (10). Simultaneous excision of bilateral lung metastases of children by one-stage LCA-VATS is complex. First, it requires 2 preoperative disinfections of the surgical area and 2 changes of body position. In addition, some children with chest wall malformations or certain medical diseases are not eligible for one-stage LCA-VATS. In a meta-analysis of 934 patients, SA-VATS did not increase the incidence of postoperative complications, intraoperative blood loss, postoperative volume of drainage, and the duration of postoperative chest tube placement compared with LCA-VATS (11). It has been shown that the length of hospital stay and the cost of hospitalization of one-stage SA-VATS are reduced compared with two-stage LCA-VATS (12). In our case, the operation via SA-VATS was performed through 3 small incisions. The child did not use any analgesic drugs after surgery, and the pain score on POD 7 in hospital was above 9 points. No chest wall complications, such as shoulder elevation, were found on measurements taken at the fourth month after surgery. The procedure was more acceptable to the parents of the child both before and after surgery, in terms of aesthetics, cost, and length of stay.
In SA-VATS, the incisions are made below the xiphoid process of the sternum, a point from where the anterior mediastinum can also be accessed. The Japanese scholar, Suda, reported the first bilateral pulmonary metastatic tumor resection via SA-VATS in an adult (13). Since then, SA-VATS in adults have mostly been performed by means of applying atmospheric pressure through a single subxiphoid incision, which partly requires a sternal retractor (14,15). After entering the pleural cavity, we first attempted to use the uniportal method to perform the operation. But under the condition of natural pneumothorax, we didn’t obtain sufficient operating space just like adults. In school-age children, the diaphragm was positioned higher due to the larger size of the liver. When attempting to use the uniportal method to remove the tumors located in the posterior basal segment of bilateral lower lobes, we found that surgical instruments had limited range of motion and interfered with each other. Hence, we used two additional incisions in a triangular arrangement with the subxiphoid incision. The 3 incisions that we designed in this case not only facilitated the establishment of the artificial pneumothorax but also the ability to operate on both left and right sides. When inserting the trocar, however, the left trocar entered the abdominal cavity, and extra time was required to repair the right diaphragm. This also suggests that the trocar should be placed parallel to and against the chest wall. Overall, trocar placement and surgical space establishment in this operation were quick and safe.
In conclusion, we have reported the first case of a pediatric patient with WT and bilateral lung metastases successfully treated via SA-VATS. Minimally invasive surgery for oncological children has been the mainstream of pediatric surgery in today’s era. The parents were very satisfied with the planning of the operation and the treatment effect. Most importantly, one-stage surgery allowed more time for postoperative adjuvant treatment in the short term. Of course, pediatric LCA-VATS is the basis of SA-VATS, and so it needs to be completed by surgeons who are skilled in thoracoscopic operation and familiar with thoracoabdominal anatomy. It also may take a longer time to learn. Further cohort studies are warranted to elucidate differences between pediatric SA-VATS and LCA-VATS.
Acknowledgments
We thank K. Gilbert and J. Jones for their linguistic assistance during the preparation of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-102/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-102/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Green DM, Lange JM, Qu A, et al. Pulmonary disease after treatment for Wilms tumor: a report from the national wilms tumor long-term follow-up study. Pediatr Blood Cancer 2013;60:1721-6. [Crossref] [PubMed]
- Spreafico F, Biasoni D, Lo Vullo S, et al. Results of the Third AIEOP Cooperative Protocol on Wilms Tumor (TW2003) and Related Considerations. J Urol 2017;198:1138-45. [Crossref] [PubMed]
- Verschuur A, Van Tinteren H, Graf N, et al. Treatment of pulmonary metastases in children with stage IV nephroblastoma with risk-based use of pulmonary radiotherapy. J Clin Oncol 2012;30:3533-9. [Crossref] [PubMed]
- De Kraker J, Tournade MF, Weirich A, et al. Wilms tumour stage IV. A report from the SIOP-9 study. Med Pediatr Oncol 1997;29:370.
- Parida L, Fernandez-Pineda I, Uffman J, et al. Thoracoscopic resection of computed tomography-localized lung nodules in children. J Pediatr Surg 2013;48:750-6. [Crossref] [PubMed]
- Kasteler R, Lichtensteiger C, Schindera C, et al. Validation of questionnaire-reported chest wall abnormalities with a telephone interview in Swiss childhood cancer survivors. BMC Cancer 2021;21:787. [Crossref] [PubMed]
- Lam FKF, Lau CT, Yu MO, et al. Comparison of thoracoscopy vs. thoracotomy on musculoskeletal outcomes of children with congenital pulmonary airway malformation (CPAM). J Pediatr Surg 2021;56:1732-6. [Crossref] [PubMed]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Mei LX, Wang YY, Chen Y, et al. Subxiphoid versus intercostal video-assisted thoracic surgery for lung resection: a meta-analysis. Minim Invasive Ther Allied Technol 2022;31:359-69. [Crossref] [PubMed]
- Negi T, Suda T, Tochii S, et al. Subxiphoid uniportal bilateral lung wedge resection. Eur J Cardiothorac Surg 2020;58:i100-2. [Crossref] [PubMed]
- Suda T, Ashikari S, Tochii S, et al. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718-9. [Crossref] [PubMed]
- Ali J, Haiyang F, Aresu G, et al. Uniportal Subxiphoid Video-Assisted Thoracoscopic Anatomical Segmentectomy: Technique and Results. Ann Thorac Surg 2018;106:1519-24. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]


