
Correlation between 5-HTTLPR gene polymorphism and cognitive function of traumatic stress in Chinese Han children
Introduction
China is a developing country with a large land area and a large population. It is seriously affected by various disasters, especially natural disasters. Further attention needs to be paid to the negative consequences of natural disasters. Experiencing natural disasters can lead to mental health problems such as anxiety, depression, or post-traumatic stress disorder (PTSD) in children and teens. Among them, PTSD is a complex disorder and the most common type of psychopathology among disaster survivors. Trauma in childhood can cause many psychological changes, and can lead to a series of psychological disorders in adulthood. The reported prevalence of diagnosable PTSD in children ranges from 30 to 60 percent (1).
As a trauma-related psychological disorder, PTSD is caused by a threat to an individual’s physical integrity which leads to a strong sense of fear or helplessness. Its clinical manifestations are characterized by re-experiencing the trauma, accompanied by emotional irritability and avoidance behavior, with serious social and family-related impacts (2,3). At the same time, studies in recent years have shown that PTSD patients exhibit significant differences in behavioral levels, including attention, executive ability, and memory (4,5). In addition, evidence has also been found on the neurophysiological mechanisms of cognitive abnormalities in PTSD patients: for example, the emotional Stroop effect study supports the pathophysiological model of PTSD, emphasizing the abnormal expression of the medial prefrontal cortex and amygdala (6,7). Bremner et al. and Wignall et al. found that the hippocampal volume of PTSD patients is generally smaller than that of normal people (8,9). These findings suggest that cognitive abnormalities in PTSD may play an important role in the occurrence, development, and outcome of PTSD, which may be a symptom of post-traumatic disease. Further research on this phenomenon will provide new perspectives for the understanding and treatment of the pathogenesis of PTSD.
When people face similar levels of trauma exposure, they are also affected by many post-traumatic factors, such as personality characteristics, cognitive patterns, social support, post-traumatic coping strategies, exposure to trauma cues, etc. However, good social support and certain genotypes are protective factors for PTSD. In addition, certain genes, such as NRG1 (10), interleukin 10 rs1800872 AA genotype (11), OXTR rs53576 genotype (12) are associated with the development of PTSD. In recent years, through modern molecular genetic techniques, several genes have been identified as associated with PTSD susceptibility, including SLC6A4, DRD2, CNR1, 5-HTT, FKBP5, and DAT1, among others (13). Serotonin is an important neurotransmitter in the body, which plays an important role in many physiological functions such as movement, feeding, reproduction, and emotion in mammals. The 5-HTT-encoding gene has been confirmed to be associated with a variety of psychiatric diseases. Currently, the polymorphisms of 5-HTTLPR and 5-HTT-VNTR related to emotion, mentality, personality disorders, and personality traits have been more closely studied (14,15). Many genetic studies on PTSD have also focused on the serotonin system, as PTSD can be treated by targeting the serotonin transporter (SLC6A4) with selective serotonin reuptake inhibitors (SSRIs) (16,17). The widely studied 5-HTTLPR variant of the SLC6A4 gene is a gene-linked polymorphic region in the 5-HTT promoter region that regulates gene expression levels (18). This variation may contribute to changes in response to SSRI treatment (19,20). To date, 5-HTTLPR has been studied in more than 300 neurological and psychiatric disorders, including PTSD (21,22), major depressive disorder (23), and Alzheimer’s disease (23). At the same time, related studies have found that the polymorphism of the 5-HTTLPR gene plays a key role in the cognitive impairment of patients with Alzheimer’s disease (24), and the cognitive control behavior contained in 5-HT 1A is also severely affected in major depressive disorder (25). A study showed (26) that the analysis of the PTSD core family found that the S allele of 5-HTT gene-linked polymorphic region (5-HTTLPR) would be preferentially transmitted to children, and the VNTR polymorphism in the second intron of the 5-HTT gene had no significant association with PTSD; the ploidy analysis showed that there were significant differences in the transmission of the 5-HTTLPR and VNTR alleles. At the same time, the study found that the L allele was preferentially transmitted to the children (27), and the nuclear family TDT study further found that there was no difference in the transmission of the 5-HTTLPR allele, but it is related to the severity of social impairment. The S allele is preferentially transmitted in individuals with severe disabilities, and the L allele is preferentially transmitted in individuals with mild-to-moderate disabilities, suggesting that the 5-HTTLPR allele itself does not determine the susceptibility to PTSD. Perceptual function may correlate with the severity of social impairment. These studies have shown that polymorphism of the 5-HTTLPR gene has adverse effects on cognitive function in patients.
Although there are many studies on 5-HTTLPR gene polymorphisms and PTSD, the subjects are mainly adults. Moreover, the research on PTSD in children in my country started relatively late, mainly focusing on the symptoms, influencing factors, assessment tools, and intervention strategies of PTSD in children. The correlation between 5-HTTLPR gene polymorphism and cognitive function in children with PTSD has not been reported yet. Combined with the actual situation in my country, this study took Han children with PTSD as the research object.
The aim of this study was to investigate the relationship between the 5-HTTLPR and cognitive dysfunction in Chinese Han children with PTSD. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-289/rc).
Methods
Research participants
Children with PTSD who sought medical treatment in The Fourth People’s Hospital of Haikou from December 2019 to December 2021 were selected as the research participants. Two experienced pediatric neurologists independently diagnosed the children with PTSD who met the criteria and allocated them to the PTSD group (60 cases). The average age was 7.52±1.23 years old, including 32 boys and 28 girls; during the same period, healthy volunteer children were matched with the normal control group (60 cases) according to the number of PTSD children, with an average age of 7.64±1.15 years old, including 35 boys and 25 girls. All 120 participants were of Han nationality. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Fourth People’s Hospital of Haikou (No. 202006) and informed consent was provided by all participants’ parents or legal guardians.
The inclusion criteria were as follows: (I) Conformance to the International Statistical Classification of Diseases and Related Health Problems (10th Edition); (II) PTSD Level Assessment Scale ≥30 points; (III) Cognitive Function Assessment Scale ≤24 points; and (IV) the first onset of PTSD. The exclusion criteria were as follows: (I) history of coma for more than 5 days; (II) clear history of neurological disease; (III) history of traumatic brain injury; (IV) history of mental illness; and (V) drug abuse.
PTSD level assessment
Patients were assessed for PTSD levels using the Children’s Revised Impact of Event Scale (CRIES), which was developed from the Impact of Event Scale - Revised (IES-R) as a 13-item screening tool for use after experiencing traumatic events for children at risk of developing PTSD. The CRIES includes assessment of aggression (4 tests), avoidance (4 tests), and arousal symptoms (5 tests), 3 of which are in compliance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) dimensions of diagnostic criteria. Each question is answered on the original four-point scale (almost never, occasionally, sometimes, often) and rated 0, 1, 3, and 5, with no reversed items within the question. In this scale, children’s total response score ranges from 0 to 65, reflecting the severity of post-traumatic stress reactions, and a cumulative score of all items ≥30 is considered positive for PTSD.
Cognitive function level assessment
The Mini-mental State Examination or Mini-mental State Examination (MMSE) is often used to screen for cognitive function (28). In assessing the cognitive function of study participants, the MMSE is more sensitive in detecting cognitive impairment than using informal questioning or general impressions of the patient. The MMSE scale provides measures of orientation, memory, recall, and language ability, and its scoring standard is the sum of the points of all correctly answered sub-items, a total of 30 items, 1 point for each item, and the total score range is 0 to 30 points. Scores ≤24 were considered to indicate cognitive impairment. The test-retest reliability was 0.80–0.99, and the inter-subject reliability was 0.95–1.00. The evaluation was performed by 2 pediatric neurologists with intermediate titles and above, with relevant clinical experience.
5-HTTLPR gene polymorphism detection
After rinsing the participants’ mouths, sterile cotton swabs were used to scrape oral mucosal epithelial cells, which were then placed in a 15 mL centrifuge tube for low-temperature storage and returned to the laboratory. An oral swab DNA extraction kit (DP322; Tiangen Biochemical Technology, Beijing, China) was used to extract the genomic DNA of oral mucosal epithelial cells and stored in a −80 ℃ refrigerator. Primer Premier 5.0 primer design software (Premier Biosoft, Palo Alto, CA, USA) was used to design polymerase chain reaction (PCR) primers (Table 1), and the primers were synthesized by Shanghai Sangon Bioengineering Co., Ltd. (Shanghai, China. The PCR amplification reaction system was 30 µL in total, containing 100 nG of genomic DNA, 1× PCR reaction buffer, 20 pmol forward primer, 4 mmol dNTPs, and 1 U TaqDNA polymerase. The PCR amplification reaction program was as follows: DNA denaturation at 94 ℃ for 5 minutes, (94 ℃ 30 s) × 35 cycles, (64 ℃ 30 s) × 1 cycle, (72 ℃ 1 min) × 1 cycle, and after the cycle, 72 ℃ extension for 5 minutes (LineGene 9600 plus; Bioer Technology Co., Ltd., Hangzhou, China). The PCR products amplified by 5-HTTLPR were separated by 2% agarose gel electrophoresis, and the results were observed and photographed using an automatic gel imaging analysis system (FDR-980A; Shanghai Forri Technology Co., Ltd., Shanghai, China). After PCR amplification, the fragments of the target gene were divided into 528 and 484 bp. The 528 bp gene fragment was the L-type allele, and the 484 bp gene fragment was the S-type allele. Through the law of inheritance, 3 genotypes could be obtained, namely, the LL, LS, and SS genotypes. As there is a clear difference between heterozygotes (LS) and homozygotes (LL/SS), and the target gene fragment is a tandem repeat sequence, the genotype of the PCR product could be directly identified from the electrophoresis gel.
Table 1
| Name | Sequence (5'-3') |
|---|---|
| 5-HTTLPR upstream primer | 5'-GGCGTTGCCGCTCTGAATGC-3' |
| 5-HTTLPR downstream primer | 5'-GAGGGACTGAGCTGGACAACCAC-3' |
Statistical analysis
Statistical analysis and graphing of data were performed using SPSS 21.0 statistical software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA, USA). Goodness of fit chi-square was used to calculate whether the distribution of 5-HTTLPR genotypes in each group conformed to Hardy-Weinberg equilibrium law. Enumeration data were expressed as percentages (%), and differences in patient characteristics and genotype frequency distribution were assessed by χ2 test (or Fisher’s exact test). All examinations were bilateral, and P<0.05 was considered statistically significant.
Results
Comparison of clinical baseline data of study participants
Preliminary analysis of clinical baseline data showed that there was no significant difference in gender and age between children with PTSD compared with children in the control group (all P>0.05); in addition, the CRIES score in the PTSD group (32.48±1.52) was significantly higher than that of the control group (18.75±1.68), and the MMSE score (18.25±1.95) was significantly lower than that of the control group (26.57±1.79) (all P<0.001). These results suggest that PTSD is associated with cognitive impairment in children, as shown in Table 2.
Table 2
| Items | PTSD group | Control group | P value |
|---|---|---|---|
| Gender, n | 0.581 | ||
| Male | 32 | 35 | |
| Female | 28 | 25 | |
| Age, years, mean ± SD | 7.52±1.23 | 7.64±1.15 | 0.323 |
| CRIES score, mean ± SD | 32.48±1.52 | 18.75±1.68 | <0.001 |
| MMSE score, mean ± SD | 18.25±1.95 | 26.57±1.79 | <0.001 |
PTSD, post-traumatic stress disorder; CRIES, Children’s Revised Impact of Event Scale; MMSE, Mini Mental State Examination.
Hardy-Weinberg equilibrium test
The S allele of 5-HTTLPR is currently thought to increase an individual’s risk of developing PTSD (29). In order to study the relationship between 5-HTTLPR gene polymorphism and PTSD in children, we first detected the 5-HTTLPR gene polymorphism in 120 children with PTSD by PCR, and then analyzed the distribution of 5-HTTLPR genotype by Hardy-Weinberg equilibrium test. The results showed that the theoretically expected numbers of LL, LS, and SS genotypes in the PTSD group were 3, 22, and 35, respectively, while the actual observed numbers of LL, LS, and SS genotypes were 4, 20, and 36, respectively. Among 36 cases, with no statistical difference between the 2 (P>0.05); for the control group, the theoretically expected numbers of LL, LS, and SS genotypes were 17, 30, and 13, respectively, while the actual observed LL, LS, and SS genotypes were 18, 28, and 14. The numbers of LL, LS and SS genotypes were 18, 28, and 14, respectively, and there was no statistical difference between them (P>0.05). The above results indicate that the genotype distribution of 5-HTTLPR conforms to Hardy-Weinberg equilibrium and is representative of the population, as shown in Table 3.
Table 3
| Groups | Genotype | Observed value | Expected value | χ2 | P value |
|---|---|---|---|---|---|
| PTSD group | LL | 4 | 3 | 0.252 | 0.882 |
| LS | 20 | 22 | |||
| SS | 36 | 35 | |||
| Control group | LL | 18 | 17 | 0.135 | 0.935 |
| LS | 28 | 30 | |||
| SS | 14 | 13 |
PTSD, post-traumatic stress disorder.
Genotyping and gene frequency distribution of 5-HTTLPR polymorphisms
Further, we analyzed the genotyping and gene frequency distribution of 5-HTTLPR gene polymorphism. Through PCR detection, the 3 genotypes of LL, LS and SS were obtained after PCR amplification (Figure 1). Subsequently, we performed statistical analysis on the frequency distribution of LL, LS, and SS genotypes in the PTSD group and the control group. The results showed that in the PTSD group, 4 cases (6.67%), 20 cases (33.3%), and 36 cases (60.00%) had LL, LS, and SS types, respectively, and in the healthy control group, there were 18 cases (30.00%), 28 cases (46.67%), and 14 cases (23.33%) of children, respectively, and there was a statistical difference between the 2 groups (P<0.01). In terms of allele frequency, the L and S genes appeared in 23.33% and 76.67% of the PTSD group, respectively, while the L and S genes in the control group appeared in 53.33% and 46.67% of the healthy control group, respectively, with a statistically significant difference between the 2 groups (P<0.01), as shown in Table 4.
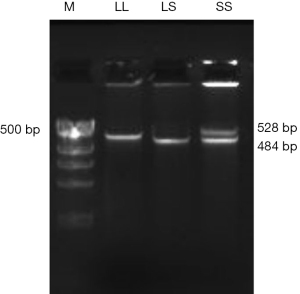
Table 4
| Groups | Genotype frequency | Allele frequency | ||||
|---|---|---|---|---|---|---|
| LL | LS | SS | L | S | ||
| PTSD group, n (%) | 4 (6.67%) | 20 (33.33%) | 36 (60.00%) | 28 (23.33%) | 92 (76.67%) | |
| Control group, n (%) | 18 (30.00%) | 28 (46.67%) | 14 (23.33%) | 64 (53.33%) | 56 (46.67%) | |
| χ2 | 19.92 | 22.84 | ||||
| P-value | <0.01 | <0.01 | ||||
PTSD, post-traumatic stress disorder.
5-HTTLPR gene polymorphism is associated with cognitive function in PTSD
A previous study has shown that the 5-HTTLPR gene polymorphism is associated with cognitive function in PTSD patients (30). In order to explore whether the 5-HTTLPR gene polymorphism is also related to cognitive impairment in children with PTSD, we analyzed the cognitive function of children with PTSD with the LL, LS, and SS genotypes. The results showed that the MMSE scores of children with the LS genotype (19.95±0.94) and SS genotype (16.94±1.37) were significantly lower than those of patients with the LL genotype (21.25±0.50) (both P<0.05). Meanwhile, the MMSE scores of children with SS genotype were also significantly lower than those of children with LS genotype (P<0.05). The above results indicate that the 5-HTTLPR genotype is associated with cognitive impairment in children with PTSD, and the S allele significantly increases the risk of cognitive impairment in children with PTSD, as shown in Table 5.
Table 5
| Genotype | N | MMSE score | Pa | Pb |
|---|---|---|---|---|
| LL | 4 | 21.25±0.50 | – | – |
| LS | 20 | 19.95±0.94 | 0.003 | – |
| SS | 36 | 16.94±1.37 | <0.001 | <0.001 |
The independent sample unpaired t-test was used for the comparison between the two groups. Pa means LS vs. LL/SS vs. LL; Pb means SS vs. LS. P<0.05 means the difference is statistically significant. PTSD, post-traumatic stress disorder.
Discussion
Stressful events such as natural disasters and man-made trauma are known to have a significant impact on people’s mental health and may lead to conditions such as PTSD and depression (31,32). In recent years, events such as the 2008 Wenchuan earthquake (33) and the 2019 novel coronavirus pneumonia (34,35), have further heightened attention and research efforts on PTSD.
To date, there have been many research reports on PTSD and its pathogenesis, but the influencing factors of genetic factors on PTSD and PTSD cognitive function are still unclear. The genetic research of PTSD is still in its infancy, and it was confirmed by Koenen et al.’s in-depth study on the gene polymorphism of FKBP5 and the hypothalamic-pituitary-adrenal (HPA) axis that the gene polymorphism of FKBP5 can participate in the HPA axis by affecting the activity of the glucocorticoid receptor, and can be used to predict the development of PTSD (36). Mustapić et al. found that the CC genotype of dopamine beta-hydroxylase (DBH), an enzyme that catalyzes the conversion of dopamine to norepinephrine, was associated with lower DBH in a subset of veterans diagnosed with PTSD. There was an association between activity, but no significant association between DBH gene polymorphisms and veterans (37). However, in a study of polymorphisms within the NPY gene, no association was found with the diagnosis of PTSD (38). In addition, another study reported that the D2A1 allele in the DRD2 gene was significantly associated with the diagnosis of PTSD (39), and another study found that the DRD2 allele was not associated with PTSD of trauma exposure, possibly with the unassessed control group (40). Since then, a significant association between the D2A1 allele and PTSD in those individuals with PTSD who engaged in harmful drinking, and the existence of different polymorphisms in the DRD2 gene and PTSD in veterans’ significant association. Another study reported a significant association between polymorphisms in the dopamine transporter SLC6A3 gene and chronic PTSD versus trauma-exposed controls (41). The genes studied above all explain the pathogenesis of PTSD through dopamine affecting the amygdala, thereby affecting the limbic-frontal neural circuit.
In addition, researchers have also found that 5-HTT plays a crucial role in the study of limbic-frontal neural circuits (42); many studies have also focused on the activation of serotonin transporter (5-HTTLPR) polymorphisms in subregions in which the short (S) allele has been shown to reduce the transcriptional efficiency of the 5-HTT gene promoter (43,44). Studies have shown that the 5-HTTLPR gene polymorphism is associated with obstructive sleep apnea (45), female obsessive-compulsive disorder (46), postpartum depression (47), anorexia nervosa (48), and autism spectrum disorder (49) closely related. In addition, one study has demonstrated an increased risk of migraine in European women with the S allele (50). The study by Lee et al. found that the SS genotype was excessive in the Korean PTSD patient population relative to the control group (51), which further promoted Kilpatrick et al. (52) to study the correlation between 5-HTTLPR gene polymorphism and PTSD. In a study of adults exposed to Florida hurricanes, those with SS genotypes were found to be at higher risk of developing PTSD among participants exposed to high-stress environments (high exposure vs. hurricane and low social support) (52). Another study in this population found a significant interaction between the 5-HTTLPR genotype and county-level environment, such that the S allele was associated with reduced PTSD risk in low-risk environments (low county-level crime and unemployment) related, but to increase the risk of PTSD in high-risk settings (53). These studies have shown that PTSD is more likely to occur in individuals with heterozygous SL or homozygous SS genes than homozygous LL genes.
Patients with PTSD patients have shown significant differences in behavioral levels, including attention, executive ability, and memory (4). This study focused on the relationship between 5-HTTLPR gene polymorphism and cognitive function in children with PTSD. Through the analysis of 5-HTTLPR gene polymorphism in 60 Han children with PTSD and 60 healthy children, it was found that there were significant differences in genotype, gene frequency, and cognitive function between the 2 groups. In addition, among the children with PTSD, the cognitive function assessment scores of the children with LS and SS genotypes were significantly lower than those of the children with the LL genotype. This indicated that the 5-HTTLPR gene polymorphism was related to the cognitive function of children with PTSD, which also indicated that the 5-HTTLPR gene polymorphism was related to PTSD. The PCR results of 5-HTTLPR showed that the proportion of the S allele was significantly more than that of the L allele (S=76.67%; L=23.33%), and the genotypes SS and LS alleles were significantly dominant, which was consistent with previously reported results. The results of the Chinese Sichuan Han population (S=85.0%; L=15.0%) (33) were basically consistent, indicating that for the Chinese Han population, the allele S and genotype SS have a higher proportion in these populations, while 5- the “S” allele of HTTLPR gene polymorphism may be an important influencing factor of cognitive dysfunction in Han PTSD patients. However, it is related to the “S” of the 5-HTTLPR gene polymorphism in the Tibetan population (S=53.1%; L=46.9%) (15) and the European and American population (S=42.86%; L=53.76%) (54). There are large differences in alleles, so the effect of ethnic background needs to be considered in the subsequent research on 5-HTTLPR gene polymorphism.
This study had some limitations. The first is that we did not analyze the relationship between genetic polymorphisms and symptom development. This may require longer follow-up of patients. Second, we did not analyze the relationship between genetic polymorphisms and environment. Elucidating gene-environment correlations can increase the clinical applicability of the conclusions. Finally, the research cohort comprised only Chinese Han children. For children of other ethnic groups or countries, the relationship between 5-HTTLPR gene polymorphism and cognitive dysfunction in PTSD patients is still unclear. At the same time, the specific loci of 5-HTTLPR gene polymorphism and the relationship between 5-HTTLPR gene polymorphism and resting-state brain functional characteristics of PTSD children were not studied. Therefore, based on the 5-HTTLPR polymorphism, further exploration of the genetic mechanism of PTSD treatment in children of other races or countries will be carried out in the future.
Acknowledgments
Funding: This study was supported by the Natural Science Foundation of Hainan Province (No. 820MS167).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-289/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-289/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-289/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dalgleish T, Moradi AR, Taghavi MR, et al. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol Med 2001;31:541-7. [Crossref] [PubMed]
- Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012;13:769-87. [Crossref] [PubMed]
- Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science 2012;338:79-82. [Crossref] [PubMed]
- Aupperle RL, Melrose AJ, Stein MB, et al. Executive function and PTSD: disengaging from trauma. Neuropharmacology 2012;62:686-94. [Crossref] [PubMed]
- Bolton C, Thilges S, Lane C, et al. Post-traumatic Stress Disorder Following Acute Delirium. J Clin Psychol Med Settings 2021;28:31-9. [Crossref] [PubMed]
- Bremner JD, Staib LH, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999;45:806-16. [Crossref] [PubMed]
- Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000;47:769-76. [Crossref] [PubMed]
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81. [Crossref] [PubMed]
- Wignall EL, Dickson JM, Vaughan P, et al. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry 2004;56:832-6. [Crossref] [PubMed]
- Luo L, Li L, Guo M, et al. Genetic variation in NRG 1 gene and risk of post-traumatic stress disorders in patients with hepatocellular carcinoma. J Clin Lab Anal 2020;34:e23187. [Crossref] [PubMed]
- Chen YL, Zheng YJ, Shen YL, et al. Male adolescents with Interleukin 10 rs1800872 AA genotype had higher prevalence and slower recoveries of post-traumatic stress disorder at late stage of a follow-up. Neurosci Lett 2022;771:136411. [Crossref] [PubMed]
- Cao C, Wang L, Wu J, et al. Association between the OXTR rs53576 genotype and latent profiles of post-traumatic stress disorder and depression symptoms in a representative sample of earthquake survivors. Anxiety Stress Coping 2020;33:140-7. [Crossref] [PubMed]
- Marshall LL, Hayslett RL. Post-traumatic Stress Disorder in Middle Age and Beyond. Sr Care Pharm 2021;36:191-207. [Crossref] [PubMed]
- Tian Y, Liu H, Guse L, et al. Association of Genetic Factors and Gene-Environment Interactions With Risk of Developing Posttraumatic Stress Disorder in a Case-Control Study. Biol Res Nurs 2015;17:364-72. [Crossref] [PubMed]
- Xiao Y, Liu D, Liu K, et al. Association of DRD2, 5-HTTLPR, and 5-HTTVNTR Gene Polymorphisms With Posttraumatic Stress Disorder in Tibetan Adolescents: A Case-Control Study. Biol Res Nurs 2019;21:286-95. [Crossref] [PubMed]
- Ramamoorthy S, Bauman AL, Moore KR, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A 1993;90:2542-6. [Crossref] [PubMed]
- Ravindran LN, Stein MB. Pharmacotherapy of post-traumatic stress disorder. Curr Top Behav Neurosci 2010;2:505-25. [Crossref] [PubMed]
- Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 2006;78:815-26. [Crossref] [PubMed]
- Lotrich FE, Pollock BG, Ferrell RE. Serotonin transporter promoter polymorphism in African Americans: allele frequencies and implications for treatment. Am J Pharmacogenomics 2003;3:145-7. [Crossref] [PubMed]
- Smits KM, Smits LJ, Peeters FP, et al. The influence of 5-HTTLPR and STin2 polymorphisms in the serotonin transporter gene on treatment effect of selective serotonin reuptake inhibitors in depressive patients. Psychiatr Genet 2008;18:184-90. [Crossref] [PubMed]
- Gressier F, Calati R, Balestri M, et al. The 5-HTTLPR polymorphism and posttraumatic stress disorder: a meta-analysis. J Trauma Stress 2013;26:645-53. [Crossref] [PubMed]
- Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One 2015;10:e0119998. [Crossref] [PubMed]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9. [Crossref] [PubMed]
- Creese B, Ballard C, Jones E. Cognitive impairment in studies of 5HTTLPR and psychosis in Alzheimer’s disease: a systematic review. Dement Geriatr Cogn Disord 2013;35:155-64. [Crossref] [PubMed]
- Langenecker SA, Mickey BJ, Eichhammer P, et al. Cognitive Control as a 5-HT1A-Based Domain That Is Disrupted in Major Depressive Disorder. Front Psychol 2019;10:691. [Crossref] [PubMed]
- Grabe HJ, Schwahn C, Mahler J, et al. Moderation of adult depression by the serotonin transporter promoter variant (5-HTTLPR), childhood abuse and adult traumatic events in a general population sample. Am J Med Genet B Neuropsychiatr Genet 2012;159B:298-309. [Crossref] [PubMed]
- Navarro-Mateu F, Escámez T, Koenen KC, et al. Meta-analyses of the 5-HTTLPR polymorphisms and post-traumatic stress disorder. PLoS One 2013;8:e66227. [Crossref] [PubMed]
- Myrberg K, Hydén LC, Samuelsson C. The mini-mental state examination (MMSE) from a language perspective: an analysis of test interaction. Clin Linguist Phon 2020;34:652-70. [Crossref] [PubMed]
- Xie P, Kranzler HR, Poling J, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry 2009;66:1201-9. [Crossref] [PubMed]
- Morey RA, Hariri AR, Gold AL, et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry 2011;11:76. [Crossref] [PubMed]
- Plexousakis SS, Kourkoutas E, Giovazolias T, et al. School Bullying and Post-traumatic Stress Disorder Symptoms: The Role of Parental Bonding. Front Public Health 2019;7:75. [Crossref] [PubMed]
- Schwartz RM, Rasul R, Gargano LM, et al. Examining Associations Between Hurricane Sandy Exposure and Posttraumatic Stress Disorder by Community of Residence. J Trauma Stress 2019;32:677-87. [Crossref] [PubMed]
- Li G, Wang L, Cao C, et al. Post-traumatic stress symptoms of children and adolescents exposed to the 2008 Wenchuan Earthquake: A longitudinal study of 5-HTTLPR genotype main effects and gene-environment interactions. Int J Psychol 2021;56:22-9. [Crossref] [PubMed]
- Liang L, Ren H, Cao R, et al. The Effect of COVID-19 on Youth Mental Health. Psychiatr Q 2020;91:841-52. [Crossref] [PubMed]
- Tang W, Hu T, Hu B, et al. Prevalence and correlates of PTSD and depressive symptoms one month after the outbreak of the COVID-19 epidemic in a sample of home-quarantined Chinese university students. J Affect Disord 2020;274:1-7. [Crossref] [PubMed]
- Koenen KC, Saxe G, Purcell S, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry 2005;10:1058-9. [Crossref] [PubMed]
- Mustapić M, Pivac N, Kozarić-Kovacić D, et al. Dopamine beta-hydroxylase (DBH) activity and -1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 2007;144B:1087-9. [Crossref] [PubMed]
- Lappalainen J, Kranzler HR, Malison R, et al. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry 2002;59:825-31. [Crossref] [PubMed]
- Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry 1996;40:368-72. [Crossref] [PubMed]
- Gelernter J, Southwick S, Goodson S, et al. No association between D2 dopamine receptor (DRD2) "A" system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biol Psychiatry 1999;45:620-5. [Crossref] [PubMed]
- Segman RH, Cooper-Kazaz R, Macciardi F, et al. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry 2002;7:903-7. [Crossref] [PubMed]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol 1999;82:69-85. [Crossref] [PubMed]
- Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527-31. [Crossref] [PubMed]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 2005;8:828-34. [Crossref] [PubMed]
- Maierean AD, Bordea IR, Salagean T, et al. Polymorphism of the Serotonin Transporter Gene and the Peripheral 5-Hydroxytryptamine in Obstructive Sleep Apnea: What Do We Know and What are We Looking for? A Systematic Review of the Literature. Nat Sci Sleep 2021;13:125-39. [Crossref] [PubMed]
- Mak L, Streiner DL, Steiner M. Is serotonin transporter polymorphism (5-HTTLPR) allele status a predictor for obsessive-compulsive disorder? A meta-analysis. Arch Womens Ment Health 2015;18:435-45. [Crossref] [PubMed]
- Yang Y, Fang M, Du X, et al. Lucky gene 5-HTTLPR and postpartum depression: A systematic review. Neuro Endocrinol Lett 2017;38:316-20. [PubMed]
- Abou Al Hassan S, Cutinha D, Mattar L. The impact of COMT, BDNF and 5-HTT brain-genes on the development of anorexia nervosa: a systematic review. Eat Weight Disord 2021;26:1323-44. [Crossref] [PubMed]
- Yang PY, Menga YJ, Li T, et al. Associations of endocrine stress-related gene polymorphisms with risk of autism spectrum disorders: Evidence from an integrated meta-analysis. Autism Res 2017;10:1722-36. [Crossref] [PubMed]
- Schurks M, Rist PM, Kurth T. 5-HTTLPR polymorphism in the serotonin transporter gene and migraine: a systematic review and meta-analysis. Cephalalgia 2010;30:1296-305. [Crossref] [PubMed]
- Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety 2005;21:135-9. [Crossref] [PubMed]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry 2007;164:1693-9. [Crossref] [PubMed]
- Koenen KC, Aiello AE, Bakshis E, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol 2009;169:704-11. [Crossref] [PubMed]
- Telch MJ, Beevers CG, Rosenfield D, et al. 5-HTTLPR genotype potentiates the effects of war zone stressors on the emergence of PTSD, depressive and anxiety symptoms in soldiers deployed to iraq. World Psychiatry 2015;14:198-206. [Crossref] [PubMed]


