
Distal foundation augmentation enhances the “Bridge” role of single traditional growing rods in the treatment of severe early-onset scoliosis: a retrospective comparative cohort study
Highlight box
Key findings
• For patients with severe early-onset scoliosis (EOS) who undergo single traditional growing rod (sTGR) treatment, distal foundation augmentation (DFA) might better maintain the deformity correction, distract the growing spine, preserve balance, and decrease the incidence of implant-related complications. The efficiency of DFA was comparable to that of the gold-standard dual traditional growing rod (dTGR) treatment.
What is known and what is new?
• Distal foundation augmentation by four pedicle screws with a cross-link can increase the spinal control provided by a dTGR.
• This manuscript investigated the efficiency of DFA in patients with severe EOS who underwent sTGR treatment.
What is the implication, and what should change now?
• The “bridge” role of sTGRs in the treatment of severe EOS was enhanced by DFA. Patients could meet the criteria for conversion to dTGR implantation earlier after a series of sTGR lengthening procedures with DFA. Further multicenter randomized controlled trials are needed for more convincing conclusions.
Introduction
Early-onset scoliosis (EOS) is defined as a curvature of the spine greater than or equal to 10° in the frontal plane occurring before the age of 10 (1). The natural history of untreated EOS involves truncal shortening and profound cardiopulmonary compromise, which may cause respiratory failure and cor pulmonale (2). The fundamental principle of EOS treatment is to maximize spinal growth and pulmonary functions while minimizing the progression of deformity leading to thoracic insufficiency syndrome (3). For patients with severe and rapidly progressing EOS, growth-friendly surgical management, such as the implantation of traditional growing rods (TGRs), is usually preferred (4,5).
Dual-rod constructs allow superior stability for and better control over the growing spine; therefore, the dual traditional growing rod (dTGR) has been considered as the gold standard of distraction-based growth-friendly implants for EOS (6). Compared with the single traditional growing rod (sTGR), the dTGR can achieve greater correction of spinal deformities, better T1–S1 growth rates, and fewer implant-related complications (7). However, the implantation of dTGR constructs may not always be feasible due to patient size and the severity of the spinal deformity. Specifically, for severe kyphoscoliosis, which is usually concomitant with a poor nutritional status, the placement of a growing rod on the convex side of the deformity is problematic, and the risk of skin breakage and implant prominence is high. Therefore, sTGR implantation is usually considered as a “bridge” treatment for 6- to 8-year-old patients with a severe spinal deformity and low body mass index (BMI) (8). Following the implantation and lengthening of an sTGR, the patient grows to a larger size with more normal spinal alignment, which allows the single rod to be converted to a dual rod construct for further treatment.
The optimal anchor configuration and the prevention of anchor failure have been the subject of much debate. Distal foundation augmentation (DFA) with four pedicle screws with a cross-link has been reported to confer the strongest stability and failure loads, increasing the ability of a dTGR to control the spine (9,10). However, research on the efficiency of DFA in sTGR treatment is still limited. This study aimed to report the radiographic parameters and complications of severe EOS patients who underwent sTGR treatment combined with DFA, and compared them to those of patients who underwent sTGR without DFA or the gold-standard dTGR treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-418/rc).
Methods
Patient cohort
This was a single-center retrospective comparative cohort study of patients with severe EOS who underwent sTGR implantation between September 2010 and December 2021. The flow chart of cohort selection is displayed in Figure 1. Patients who initially underwent sTGR implantation and were followed-up for a minimum of 24 months were included. The follow-up procedures included physical examination, back skin inspection, and standing full-length spine radiographs in the outpatient department of our hospital every 6 months. The indication for sTGR implantation is typically a magnitude of the major curve greater than 80°. The sTGRs were lengthened periodically, usually for 6 to 12 months. Once the patient size, nutritional status, and severity of the spinal deformity had improved during the lengthening period, the sTGR construct was converted to a dual rod instrumentation for further growing or definitive spinal fusion. Patients who matched with either of the following criteria were excluded: (I) a previous history of spinal surgery; and (II) underwent convention surgery to a dual rod instrumentation within 24 months after the initial surgery. The sTGR cohort was further divided into two groups, differentiated by whether DFA was performed. In our center, patients who were admitted for sTGR implantation after 2018 also routinely underwent DFA.
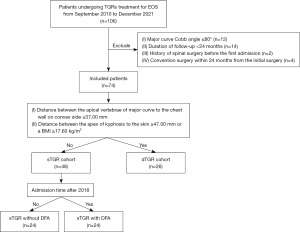
To compare the efficiency of sTGR with DFA with that of the gold standard, another cohort of patients who initially underwent dTGR implantation and were followed up for a minimum of 24 months was also retrieved. The indication for dTGR implantation or conversion from sTGR to dTGR were as follows: (I) the distance between the apical vertebrae of the major curve to the chest wall on the convex side was greater than or equal to 37.00 mm; and (II) the distance between the apex of the kyphosis to the skin was greater than or equal to 47.00 mm or the patient had a BMI greater than or equal to 17.60 kg/m2. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of Beijing Chao-Yang Hospital (No. 2022-01-21-3). The patients’ parents or legal guardians were aware of and agreed to this study, and signed the relevant informed consent.
Surgical technique
Under general anesthesia, the patients were placed in a prone position on the operating table. Two incisions were made at the upper and lower ends of scoliosis. Proximal and distal anchor points were exposed in a subperiosteal fashion. The proximal anchor points were instrumented with pedicle screws, hooks, or a hybrid of the two. For the sTGR cohort, unilateral instrumentation was used at the proximal anchor points, whereas bilateral instrumentation was applied for the dTGR cohort. In general, pedicle screws were inserted in the distal anchor points. The vertebrae cranial to the upper thoracic curve and the stable vertebrae were usually selected as the upper instrumented vertebrae and the lower instrumented vertebrae, respectively. Bone grafts were implanted in the anchor areas. The rods were generally bent for consistency with the curvature of the physiological kyphosis, and then implanted intramuscularly and fixed to the anchor points. A connector was used to connect the rods, composing the TGR. After TGR implantation, proper distraction was performed. The DFA involved four pedicle screws being placed in the distal anchor points (two adjacent levels), and the screws opposite the sTGR were connected by a short rod. A horizontal rod-to-rod crosslink was used to connect the short rod and the sTGR for the purpose of enhancing stability.
Baseline clinical characteristics
The baseline clinical characteristics of the patients before the initial surgery were collected, including their age at the initial surgery, sex, BMI, the number of lengthening procedures, and the duration of follow-up. The etiology of EOS and the type of major curve were also recorded. The classifications of etiology included idiopathic, congenital, neuromuscular, and syndromic scoliosis (11). Based on the location of the apical vertebra, the types of major curve were categorized as thoracic (T2 to T11/12), thoracolumbar (T12 to L1), and lumbar curves (L1/2 to L5) (12).
Radiographic evaluation
The magnitude of the major curve, apical vertebral translation (AVT), T2–5 thoracic kyphosis (TK), T5–12 TK, the maximal kyphosis (MK), the thoracic height (T1–T12), the spinal height (T1–S1), the distance between the C7 plumb line and the central sacral vertical line (C7PL-CSVL), and the sagittal vertical axis (SVA) were measured from standing full-length spine radiographs. The distal foundation (DF) tilt—defined as the angle between the endplate of the DF and the horizontal line—was also recorded (Figure 2). Each measure was collected preoperatively, immediately after surgery, and at the last follow-up before conversion to a dual rod instrumentation.
Statistical analysis
All statistical analyses were performed using SPSS version 25.0 (Chicago, IL, USA). Continuous variables were tested for baseline comparability between the groups using one-way analysis of variance (ANOVA). Categorical variables were tested for baseline comparability with the chi-squared test or Fisher’s exact test. Similarly, for primary clinical outcomes, one-way ANOVA was used to compare continuous variables between the groups; comparisons between time points were performed by repeated-measures ANOVA. Categorical variables were compared using the chi-squared test or Fisher’s exact test. Post hoc Bonferroni correction was applied for multiple comparisons. Correlations between outcome values of different variables were evaluated using Pearson’s correlation coefficient. A two-sided P value of less than 0.05 was considered statistically significant.
Results
Patient demographics and baseline clinical characteristics
A total of 74 patients (39 males and 35 females) with severe EOS were included in this study. There were 48 and 26 patients recruited into the sTGR and dTGR cohorts, respectively. Twenty-four patients in the sTGR cohort had undergone DFA. The mean age of all patients at the initial TGR implantation was 8.14±1.30 years. The mean BMI was 17.41±2.57 kg/m2. There were 45 (60.8%), 16 (21.6%), 9 (12.2%), and 4 (5.4%) patients with idiopathic, congenital, neuromuscular, and syndromic scoliosis, respectively. The types of major curve included main thoracic (61, 82.4%), thoracolumbar (9, 12.2%), and lumbar (4, 5.4%) curves.
The mean follow-up period after initial TGR implantation was 27.17±3.22 months. All sTGR constructs were implanted on the concave side of the major curve. An average of 2.45±0.81 lengthening procedures were performed on each patient during the follow-up period. The distal anchor points were all pedicle screws. Illustrative cases of patients who underwent sTGR implantation, sTGR implantation with DFA, and dTGR implantation are shown in Figure 3. The baseline clinical characteristics of the patients before the initial surgery are shown in Table 1. There were no significant differences between the DFA, non-DFA, and dTGR groups in terms of age at the initial surgery (7.92±1.28 vs. 8.21±1.18 vs. 8.27±1.43 years, P=0.602), sex (P=0.366), BMI (16.69±3.53 vs. 16.92±3.18 vs. 18.52±2.77 kg/m2, P=0.208), etiology (P=0.732), the type of major curve (P=0.960), the number of lengthening procedures (2.33±0.76 vs. 2.46±0.83 vs. 2.54±0.86, P=0.676), and the duration of follow-up (26.78±2.88 vs. 28.18±3.01 vs. 26.58±3.57 months, P=0.357).
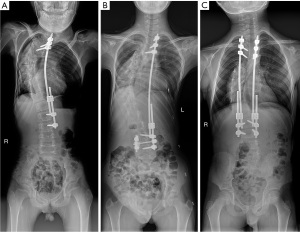
Table 1
| Variable | Overall (n=74) | sTGR cohort (n=48) | dTGR cohort (n=26) | P value | |||
|---|---|---|---|---|---|---|---|
| DFA group (n=24) | Non-DFA group (n=24) | Among groups | DFA vs. Non-DFA | DFA vs. dTGR | |||
| Age (years) | 8.14±1.30 | 7.92±1.28 | 8.21±1.18 | 8.27±1.43 | 0.602 | 0.441 | 0.343 |
| Sex | 0.366 | 0.282 | 0.405 | ||||
| Male | 39 (52.7) | 13 (54.2) | 10 (41.7) | 16 (61.5) | |||
| Female | 35 (47.3) | 11 (45.8) | 14 (58.3) | 10 (38.5) | |||
| BMI, kg/m2 | 17.41±2.57 | 16.69±3.53 | 16.92±3.18 | 18.52±2.77 | 0.208 | 0.607 | 0.135 |
| Etiology | 0.732 | 0.463 | 0.959 | ||||
| Idiopathic | 45 (60.8) | 14 (58.3) | 15 (62.5) | 16 (61.5) | |||
| Congenital | 16 (21.6) | 5 (20.8) | 7 (29.2) | 4 (15.4) | |||
| Neuromuscular | 9 (12.2) | 3 (12.5) | 2 (8.3) | 4 (15.4) | |||
| Syndromic | 4 (5.4) | 2 (8.3) | 0 (0.0) | 2 (7.7) | |||
| Major curve | 0.960 | 0.836 | 0.992 | ||||
| Thoracic | 61 (82.4) | 20 (83.3) | 19 (79.2) | 22 (84.6) | |||
| Thoracolumbar | 9 (12.2) | 3 (12.5) | 3 (12.5) | 3 (11.5) | |||
| Lumbar | 4 (5.4) | 1 (4.2) | 2 (8.3) | 1 (3.8) | |||
| Follow-up (months) | 27.17±3.22 | 26.78±2.88 | 28.18±3.01 | 26.58±3.57 | 0.357 | 0.097 | 0.752 |
| No. of lengthening procedures | 2.45±0.81 | 2.33±0.76 | 2.46±0.83 | 2.54±0.86 | 0.676 | 0.599 | 0.731 |
Data are presented as number (%) or mean ± standard deviation. DFA, distal foundation augmentation; BMI, body mass index; sTGR, single traditional growing rod; dTGR, dual traditional growing rod.
Radiographic parameters
Coronal and sagittal alignment
There was no significant difference in the preoperative major curve magnitude between the DFA, non-DFA, and dTGR groups (102.50°±17.71° vs. 93.60°±21.87° vs. 95.74±19.15, P = 0.267) (Table 2). Following TGR implantation, the curve was corrected to 55.71°±19.12° in the DFA group, 60.64°±19.90° in the non-DFA group, and 52.32°±18.01° in the dTGR group (P=0.306), with comparable correction rates of 45.2%, 36.9%, and 46.2%, respectively. However, at the last follow-up, the correction in the DFA group was maintained better than that in the non-DFA group (59.47°±18.81° vs. 70.88°±19.72°, P=0.037), and the correction rate was higher (40.6% vs. 24.0%, P=0.001). There was no significant difference in the major curve (P=0.628) or the correction rate (P=0.917) between the DFA and dTGR groups at the last follow-up.
Table 2
| Parameters | sTGR cohort (n=48) | dTGR Cohort (n=26) | P value | |||
|---|---|---|---|---|---|---|
| DFA Group (n=24) |
Non-DFA Group (n=24) | Between groups | DFA vs. Non-DFA |
DFA vs. dTGR | ||
| Coronal and sagittal alignment | ||||||
| Major curve (°) | ||||||
| Pre OP | 102.50±17.71 | 93.60±21.87 | 95.74±19.15 | 0.267 | 0.121 | 0.228 |
| Post OP | 55.71±19.12a | 60.64±19.90a | 52.32±18.01a | 0.306 | 0.372 | 0.531 |
| FU | 59.47±18.81a | 70.88±19.72ab | 56.90±17.41a | 0.024 | 0.037 | 0.628 |
| Correction rate (post vs. pre OP) | 45.2%±18.8% | 36.9%±15.2% | 46.2%±17.9% | 0.131 | 0.104 | 0.840 |
| Correction rate (FU vs. pre OP) | 40.6%±20.4% | 24.0%±17.1% | 41.1%±17.6% | 0.001 | 0.001 | 0.917 |
| Maximal kyphosis (°) | ||||||
| Pre OP | 93.30±32.16 | 83.64±28.40 | 88.35±28.55 | 0.533 | 0.264 | 0.558 |
| Post OP | 50.08±15.50a | 52.24±18.32a | 47.92±18.20a | 0.681 | 0.669 | 0.661 |
| FU | 54.67±14.89a | 65.54±19.63ab | 50.82±16.48a | 0.010 | 0.031 | 0.428 |
| Correction rate (post vs. pre OP) | 46.9%±13.0% | 41.0%±15.5% | 44.0%±16.0% | 0.398 | 0.176 | 0.501 |
| Correction rate (FU vs. pre OP) | 42.2%±19.3% | 22.0%±15.0% | 39.1%±17.3% | 0.001 | 0.001 | 0.519 |
| T2–5 thoracic kyphosis (°) | ||||||
| Pre OP | 22.17±11.54 | 23.99±9.75 | 14.34±10.83 | 0.005 | 0.559 | 0.012 |
| Post OP | 17.58±9.55 | 20.09±10.96 | 15.15±10.71 | 0.254 | 0.408 | 0.413 |
| FU | 27.11±13.35b | 31.01±13.29b | 23.99±14.04ab | 0.154 | 0.289 | 0.386 |
| T5–12 thoracic kyphosis (°) | ||||||
| Pre OP | 69.90±32.07 | 62.65±28.12 | 72.69±28.64 | 0.474 | 0.400 | 0.740 |
| Post OP | 41.80±13.84a | 36.44±15.19a | 39.54±13.43a | 0.425 | 0.194 | 0.575 |
| FU | 45.83±14.24a | 43.54±16.79a | 40.73±15.90a | 0.518 | 0.615 | 0.255 |
| Coronal and sagittal alignment | ||||||
| C7PL-CSVL (mm) | ||||||
| Pre OP | 28.77±19.19 | 34.02±20.14 | 25.02±19.56 | 0.275 | 0.358 | 0.503 |
| Post OP | 22.76±16.39 | 26.10±17.38 | 23.67±18.07 | 0.788 | 0.507 | 0.854 |
| FU | 21.00±17.01 | 31.21±17.84 | 18.13±14.88 | 0.018 | 0.036 | 0.543 |
| AVT (mm) | ||||||
| Pre OP | 88.36±27.21 | 94.60±29.40 | 70.99±24.07 | 0.008 | 0.425 | 0.026 |
| Post OP | 42.49±18.78a | 52.59±21.96a | 34.66±16.58a | 0.006 | 0.072 | 0.153 |
| FU | 45.50±21.14a | 58.91±23.25a | 37.10±20.76a | 0.003 | 0.036 | 0.177 |
| DF tilt (°) | ||||||
| Pre OP | 37.87±10.45 | 35.14±11.11 | 37.35±12.81 | 0.684 | 0.415 | 0.874 |
| Post OP | 10.30±6.64 | 14.64±7.88 | 9.06±5.57 | 0.013 | 0.029 | 0.518 |
| FU | 12.73±7.40 | 17.97±8.53 | 10.17±6.89 | 0.002 | 0.020 | 0.240 |
| SVA (mm) | ||||||
| Pre OP | 3.61±41.50 | −10.79±42.23 | 12.55±35.03 | 0.119 | 0.212 | 0.428 |
| Post OP | 21.92±37.19 | 25.32±40.05a | −13.92±47.28a | 0.002 | 0.779 | 0.003 |
| FU | 20.61±35.65 | 28.20±41.58a | 19.22±32.85b | 0.657 | 0.477 | 0.894 |
| Thoracic and spinal height | ||||||
| T1–12 height (mm) | ||||||
| Pre OP | 143.46±21.92 | 136.88±20.57 | 141.96±21.25 | 0.532 | 0.287 | 0.804 |
| Post OP | 188.52±23.83a | 177.20±20.39a | 192.66±21.97a | 0.046 | 0.081 | 0.510 |
| FU | 203.75±24.57ab | 189.19±23.94a | 205.80±23.86ab | 0.029 | 0.032 | 0.771 |
| T1–S1 height (mm) | ||||||
| Pre OP | 232.15±29.86 | 223.34±27.68 | 239.16±28.53 | 0.157 | 0.291 | 0.391 |
| Post OP | 316.18±32.63a | 300.49±30.22a | 320.78±32.06a | 0.069 | 0.090 | 0.610 |
| FU | 340.44±28.99ab | 322.01±31.55ab | 344.10±32.71ab | 0.034 | 0.044 | 0.680 |
Data are presented as mean ± standard deviation. a, significantly different from the preoperative value; b, significantly different from the postoperative value (P<0.05). sTGR, single traditional growing rod; dTGR, dual traditional growing rod; DFA, distal foundation augmentation; OP, operation; FU, follow-up; C7PL-CSVL, distance between the C7 plumb line and the central sacral vertical line; AVT, apical vertebral translation; DF, distal foundation; SVA, sagittal vertical axis.
The preoperative MK did not differ significantly between the groups (93.30°±32.16° vs. 83.64°±28.40° vs. 88.35°±28.55°, P=0.533), and after surgery, it improved to 50.08°±15.50° in the DFA group, 52.24°±18.32° in the non-DFA group, and 47.92°±18.20° in the dTGR group (P=0.681), with similar correction rates of 46.9%, 41.0%, and 44.0%, respectively. The non-DFA group experienced a significant loss of MK correction during the lengthening period (P=0.005). At the last follow-up, the MK was significantly reduced in the DFA group compared to the non-DFA group (54.67°±14.89° vs. 65.54°±19.63°, P=0.031), with a higher correction rate in the DFA group (42.2% vs. 22.0%, P=0.001). There was no significant difference in the MK (P=0.428) or the correction rate (P=0.519) between the DFA and dTGR groups at the last follow-up, which indicated that DFA was effective in preventing the progression of kyphosis during the lengthening period.
Coronal and sagittal balance
The C7PL-CSVL in the three groups was similar before (28.77±19.19 vs. 34.02±20.14 vs. 25.02±19.56, P=0.275) and after (22.76±16.39 vs. 26.10±17.38 vs. 23.67±18.07, P=0.788) TGR implantation. During the lengthening period, coronal balance was maintained well in the DFA group, but significant decompensation was observed in the non-DFA group (21.00±17.01 vs. 31.21±17.84 mm, P=0.036). At the last follow-up, similar results were found for AVT (45.50±21.14 vs. 58.91±23.25 mm, P=0.036). Compared with the non-DFA group, the DFA group had significantly better horizontalization of the DF after sTGR implantation (10.30°±6.64° vs. 14.64°±7.88°, P=0.029) and at the last follow-up (12.73°±7.40° vs. 17.97°±8.53°, P=0.020). Pearson’s correlation analysis detected a positive correlation between the post-implantation DF tilt and the C7PL-CSVL at the last follow-up (r=0.622, P=0.006). There was no significant difference in the C7PL-CSVL (P=0.543), AVT (P=0.177), or DF tilt (P=0.240) between the DFA and dTGR groups at the last follow-up.
Regarding sagittal balance, there were no significant difference in the SVA preoperatively (3.61±41.50 vs. −10.79±42.23 mm, P=0.212), immediately postoperatively (21.92±37.19 vs. 25.32±40.05 mm, P=0.779), or at the last follow-up (20.61±35.65 vs. 28.20±41.58 mm, P=0.477) between the DFA and non-DFA groups. Although the post-implantation SVA was significantly reduced in the dTGR group (P=0.002) compared to the other two groups, no significant difference was observed between the DFA and dTGR groups preoperatively (P=0.428) or at the last follow-up (P=0.894).
Thoracic and spinal height
The preoperative thoracic height was similar in all three groups (143.46±21.92 vs. 136.88±20.57 vs. 141.96±21.25 mm, P=0.532). Following sTGR implantation, the thoracic height in the DFA group increased to 188.52±23.83 mm, which was higher than that in the non-DFA group, although there was no statistical significance between the groups (P=0.081). At the last follow-up, the thoracic height observed in the DFA group was significantly greater than that in the non-DFA group (203.75±24.57 vs. 189.19±23.94 mm, P=0.032). During the lengthening period, the annual T1–T12 growth was slightly greater in the DFA group (6.79 mm/year) than the non-DFA group (5.22 mm/year).
Similar to the thoracic height, the preoperative spinal height was comparable between the three groups (232.15±29.86 vs. 223.34±27.68 vs. 239.16±28.53 mm, P=0.157). After sTGR implantation, the spinal height increased to 316.18±32.63 mm in the DFA group and 300.49±30.22 mm in the non-DFA group (P=0.090). At the last follow-up, the spinal height was significantly greater in the DFA group than in the non-DFA group (340.44±28.99 vs. 322.01±31.55 mm, P=0.044). The annual T1–S1 growth was slightly greater in the DFA group (10.78 mm/year) than in the non-DFA group (9.22 mm/year). At the last follow-up, there was no significant difference in the thoracic (P=0.771) or spinal height (P=0.680) between the DFA and dTGR groups.
Wound-related, alignment-related, and implant-related complications
A total of 58 complications (5 wound related, 17 alignment related, and 36 implant related) were noted in 35 patients. In the DFA, non-DFA, and dTGR groups, 37.5%, 54.2%, and 26.9% of patients, respectively, had at least one implant-related complication. The mean number of implant-related complications per patient was 0.42 (0–2), 0.75 (0–2), and 0.31 (0–1) in the DFA, non-DFA, and dTGR groups, respectively. There was no significant difference in the rates of wound-related (4.2% vs. 8.3% vs. 7.7%, P=0.824) and alignment-related (20.8% vs. 29.2% vs. 19.2%, P=0.674) complications between the groups. However, compared with the non-DFA group, the DFA group had a significantly lower incidence of implant-related complications (41.7% vs. 75.0%, P=0.019), especially at the DF (8.3% vs. 33.3%, P=0.033), which was similar to that in the dTGR group (Table 3).
Table 3
| Complications | sTGR Cohort (n=48) | dTGR cohort (n=26) | P value | |||
|---|---|---|---|---|---|---|
| DFA group (n=24) | Non-DFA group (n=24) | Between groups | DFA vs. Non-DFA | DFA vs. dTGR | ||
| Wound-related | 1 (4.2) | 2 (8.3) | 2 (7.7) | 0.824 | 0.551 | 0.600 |
| Deep SSI | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0.348 | 0.312 | 1.000 |
| Superficial SSI | 1 (4.2) | 1 (4.2) | 2 (7.7) | 0.815 | 1.000 | 0.600 |
| Alignment-related | 5 (20.8) | 7 (29.2) | 5 (19.2) | 0.674 | 0.505 | 0.887 |
| PJK | 4 (16.7) | 6 (25.0) | 5 (19.2) | 0.762 | 0.477 | 0.814 |
| DJK | 1 (4.2) | 1 (4.2) | 0 (0.0) | 0.573 | 1.000 | 0.293 |
| Implant-related | 10 (41.7) | 18 (75.0) | 8 (30.8) | 0.005 | 0.019 | 0.423 |
| Rod fracture | 5 (20.8) | 9 (37.5) | 3 (11.5) | 0.089 | 0.204 | 0.370 |
| Screw pull-out | 2 (8.3) | 2 (8.3) | 1 (3.8) | 0.764 | 1.000 | 0.504 |
| Screw loosening | 1 (4.2) | 4 (16.7) | 1 (3.8) | 0.174 | 0.156 | 0.954 |
| Hook dislodgment | 1 (4.2) | 2 (8.3) | 3 (11.5) | 0.634 | 0.551 | 0.337 |
| UIV endplate fracture | 1 (4.2) | 1 (4.2) | 0 (0.0) | 0.573 | 1.000 | 0.293 |
| Implant-related at DF | 2 (8.3) | 8 (33.3) | 2 (7.7) | 0.022 | 0.033 | 0.933 |
| Rod fracture | 2 (8.3) | 4 (16.7) | 1 (3.8) | 0.294 | 0.383 | 0.504 |
| Screw pull-out | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0.348 | 0.312 | 1.000 |
| Screw loosening | 0 (0.0) | 3 (12.5) | 1 (3.8) | 0.145 | 0.074 | 0.332 |
Data are presented as number (%). DFA, distal foundation augmentation; DF, distal foundation; sTGR, single traditional growing rod; dTGR, dual traditional growing rod; SSI, surgical site infection; PJK, proximal junctional kyphosis; DJK, distal junctional kyphosis; UIV, upper instrumented vertebrae.
Discussion
The concave sTGR can be considered as a starting construct which permits the transition of patients with severe EOS to the gold-standard dTGR treatment (6,8). Unlike with the dTGR construct, inadequate deformity correction, maintenance of spinal balance, and complications management cannot be ignored with the sTGR (7). There is no doubt that instrumentation with stronger biomechanical characteristics can achieve more powerful spinal control. Dissipation of mechanical stress is also important when the spine is instrumented but not fused, because the construct incurs continued loading and micromotion, which makes the implants susceptible to fatigue and mechanical failure (13). The optimal distal anchor configurations have been subject to much debate, and a foundation composed of four pedicle screws with a cross-link has been reported to confer the strongest stability and failure loads (9). Although this type of DFA had been used in dTGR procedures, whether DFA can enhance the “bridge” role of sTGRs in the treatment of severe EOS remained unknown (14). In the current study, there were no significant differences in baseline clinical characteristics, most of the preoperative radiographic parameters, the duration of treatment and follow-up, or the number of lengthening procedures between the DFA, non-DFA, and dTGR groups. Therefore, under the premise of baseline comparability, we considered that it may be rational and clinically meaningful to directly compare the radiographic outcomes and complications at the final follow-up between the three groups, for the purpose of better demonstrating the efficiency of DFA.
Growing rods placed within the pediatric spine act as an “internal brace”, which allows the continued growth of the unfused vertebras while maintaining correction and preventing curve progression (15). With the same implantation materials, mechanical stress is the sole factor impacting rod deformation. After sTGR implantation, there was no significant difference in the major curve or the MK correction rate between the groups. The maximum correction of spinal deformity has been reported to occur during the initial implantation of a TGR (16). After that, the spinal malalignment fluctuates with the lengthening, and the final fusion can only obtain a modest correction due to autofusion and spinal rigidity caused by distractions (17). Therefore, evaluating the outcome of correction maintenance is of paramount importance for TGR treatment. During the lengthening procedures, the correction was well maintained in the DFA group (Figure 4); however, a significant loss of correction in both the coronal and sagittal planes was detected in the non-DFA group, which led to a significantly lower correction rate at the last follow-up. One plausible explanation was that DFA reduced the micromotion in the foundation and dissipated the mechanical stress on the rods compared with unilateral screw fixation. The more solid foundation could provide the rods with more stuffiness to alleviate the deformation caused by curve progression and allow the application of sequential correction in the subsequent treatment. Also, the T1–T12 and T1–S1 heights in the DFA group and the dTGR group were comparable after a series of lengthening procedures, and were significantly greater than those in the non-DFA group. The greater thoracic and spinal height growth in the DFA group indicated that DFA could help the sTGR distract the abnormally growing thorax and trunk more efficiently, which in turn reduced the risk of spontaneous fusion at the uninstrumented segments. Additionally, the current study found that 66.7% of patients in the DFA group met the criteria for conversion to dTGR implantation at the last follow-up, which was significantly greater than the proportion in the non-DFA group (37.5%; P=0.043). This result indicates that DFA did indeed enhance the “bridge” role of sTGRs in the treatment of severe EOS.

Coronal imbalance is a common complication of sTGR implantation during the lengthening period that cannot be spontaneously compensated (18). To correct severe coronal imbalance, radical lumbar osteotomy and extending pelvic fixation are usually needed in the final fusion surgery, which can cause high-level surgical trauma and may increase the risk of postoperative complications, impacting patients’ long-term quality of life (19). Thus, evaluating the clinical outcome of coronal balance during follow-up is important. In the current study, the DFA group showed better maintenance of coronal balance. Several studies have reported DF horizontalization to be an important procedure for restoring or maintaining coronal balance after instrumentation because it is located in or close to the stable area (18,20,21). Before the initial surgery, we observed no significant difference in the DF tilt between the DFA and non-DFA groups (37.87°±10.45° vs. 35.14°±11.11°, P=0.415); however, a more residual oblique DF was observed in the non-DFA group after sTGR implantation (10.30°±6.64° vs. 14.64°±7.88°, P=0.029) and at the last follow-up (12.73°±7.40° vs. 17.97°±8.53°, P=0.020). Because EOS treatment requires repeated construct lengthening, if the initial surgery cannot achieve horizontal placement of the DF, the patient is at a high risk of coronal imbalance during the lengthening procedure. Compared with a unilateral screw, bilateral screws in DFA can tolerate larger correction forces, allowing the surgeon to restore a horizontal foundation through distraction, compression, and de-rotation. The cross-link also provides the foundation with more stability, making it less susceptible to becoming oblique during goring rod distraction.
The complications rate of TGR treatment in patients with EOS has been reported to be as high as 50% (13,22,23). One of the major concerns of TGR implantation is the risk of implant failure, specifically at the bone-implant interface (e.g., screw pull-out, screw loosening, hook dislodgment), and rod fracture. Compared with adolescents, younger children usually have less soft-tissue coverage and more pliable cortical bone in the pedicles as well as in the dorsal lamina. As a result, implant migration or breakage is not uncommon (24,25). The implant-related complications rates for the different etiologic subtypes of EOS, based on the multicenter Growing Spine Study Group database, have been reported to range from 46.2–60.7% (26). The overall implant-related complications rate was 48.6% for all patients in the current study and 58.3% in the sTGR cohort. Our finding that implant-related complications occurred frequently and recurrently among patients who received sTGR treatment is not unexpected. The incidence of such complications reached 75.0% in the non-DFA group; however, when DFA was performed, the incidence was significantly decreased to 41.7%, which was a similar to result to that of the gold-standard dTGR treatment (30.8%). Screw pull-out/loosening was frequently observed at the distal anchor points, and the most common location of rod fracture was adjacent to the DF (27). As mentioned above, we anticipated that DFA would dissipate the mechanical stress in the rods and screws, decreasing the implant loads, micromotion, and consequent failure at the DF. In the current study, the DFA group did present with a significantly lower implant-related complications rate at the DF, which demonstrated the stronger biomechanical characteristics provided by DFA and is consistent with the results of an earlier finite element analysis (10).
This study had several limitations. First, the duration of follow-up for the patients with EOS was too short. The cohorts will be re-analyzed in the future, ideally after definitive spinal fusion, to determine subsequent outcomes and complications. Second, we have attributed the better clinical outcomes of DFA to its biomechanical characteristics; however, this is only a hypothesis, and a further cadaveric test is needed for verification. Third, the diversity of the etiologies and curve types among the patients may have impacted the reliability of our results. Finally, due to the retrospective study design and limited sample size, further multicenter prospective randomized controlled trials with longer follow-up periods should be performed to obtain more convincing conclusions.
Conclusions
For patients with severe EOS who undergo sTGR treatment, DFA might better maintain deformity correction, distract the growing thorax and trunk, preserve balance, and decrease the incidence of implant-related complications, especially at the DF. The efficiency of sTGR with DFA was comparable to that of the gold-standard dTGR treatment. Patients could meet the criteria for the conversion to dTGR earlier after a series of sTGR lengthening with DFA. However, due to the retrospective design and limited sample size of our study, further multicenter randomized controlled trials should be performed for more convincing conclusions.
Acknowledgments
We thank Dr. Ziyang Liu for guidance in data analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at available at https://tp.amegroups.com/article/view/10.21037/tp-22-418/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-418/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-418/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of Beijing Chao-Yang Hospital (No. 2022-01-21-3). The patients’ parents or legal guardians were aware of and agreed to this study, and signed the relevant informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang S, Andras LM, Redding GJ, et al. Early-Onset Scoliosis: A Review of History, Current Treatment, and Future Directions. Pediatrics 2016;137:e20150709. [Crossref] [PubMed]
- Karol LA. The Natural History of Early-onset Scoliosis. J Pediatr Orthop 2019;39:S38-43. [Crossref] [PubMed]
- Campbell RM Jr, Smith MD. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg Am 2007;89:108-22. [PubMed]
- Zhang YB, Zhang JG. Treatment of early-onset scoliosis: techniques, indications, and complications. Chin Med J (Engl) 2020;133:351-7. [Crossref] [PubMed]
- Bekmez S, Dede O, Yazici M. Advances in growing rods treatment for early onset scoliosis. Curr Opin Pediatr 2017;29:87-93. [Crossref] [PubMed]
- Thompson GH, Akbarnia BA, Kostial P, et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine (Phila Pa 1976) 2005;30:2039-44. [Crossref] [PubMed]
- Xu GJ, Fu X, Tian P, et al. Comparison of single and dual growing rods in the treatment of early onset scoliosis: a meta-analysis. J Orthop Surg Res 2016;11:80. [Crossref] [PubMed]
- Luhmann SJ, Skaggs DL, Pahys J, et al. Single distraction-rod constructs in severe early-onset scoliosis: Indications and outcomes. Spine J 2022;22:305-12. [Crossref] [PubMed]
- Mahar AT, Bagheri R, Oka R, et al. Biomechanical comparison of different anchors (foundations) for the pediatric dual growing rod technique. Spine J 2008;8:933-9. [Crossref] [PubMed]
- Hai Y, Pan AX, Li YG, et al. Biomechanical effects on adjacent segments of different growing-rod fixation in early onset scoliosis. Zhonghua Yi Xue Za Zhi 2017;97:3768-73. [PubMed]
- Ramo BA, McClung A, Jo CH, et al. Effect of Etiology, Radiographic Severity, and Comorbidities on Baseline Parent-Reported Health Measures for Children with Early-Onset Scoliosis. J Bone Joint Surg Am 2021;103:803-11. [Crossref] [PubMed]
- Dang NR, Moreau MJ, Hill DL, et al. Intra-observer reproducibility and interobserver reliability of the radiographic parameters in the Spinal Deformity Study Group's AIS Radiographic Measurement Manual. Spine (Phila Pa 1976) 2005;30:1064-9. [Crossref] [PubMed]
- Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am 2010;92:2533-43. [Crossref] [PubMed]
- Chiba T, Inami S, Moridaira H, et al. Growing rod technique with prior foundation surgery and sublaminar taping for early-onset scoliosis. J Neurosurg Spine 2020; Epub ahead of print. [Crossref] [PubMed]
- Kocyigit IA, Olgun ZD, Demirkiran HG, et al. Graduation Protocol After Growing-Rod Treatment: Removal of Implants without New Instrumentation Is Not a Realistic Approach. J Bone Joint Surg Am 2017;99:1554-64. [Crossref] [PubMed]
- Ahuja K, Ifthekar S, Mittal S, et al. Is Final Fusion Necessary for Growing-Rod Graduates: A Systematic Review and Meta-Analysis. Global Spine J 2023;13:209-18. [Crossref] [PubMed]
- Cahill PJ, Marvil S, Cuddihy L, et al. Autofusion in the immature spine treated with growing rods. Spine (Phila Pa 1976) 2010;35:E1199-203. [Crossref] [PubMed]
- Xu L, Sun X, Wang M, et al. Coronal imbalance after growing rod treatment in early-onset scoliosis: a minimum of 5 years' follow-up. J Neurosurg Spine 2021; Epub ahead of print. [Crossref] [PubMed]
- Jain A, Hassanzadeh H, Strike SA, et al. Pelvic Fixation in Adult and Pediatric Spine Surgery: Historical Perspective, Indications, and Techniques: AAOS Exhibit Selection. J Bone Joint Surg Am 2015;97:1521-8. [Crossref] [PubMed]
- Shetty AP, Suresh S, Aiyer SN, et al. Radiological factors affecting post-operative global coronal balance in Lenke 5 C scoliosis. J Spine Surg 2017;3:541-7. [Crossref] [PubMed]
- Mannem A, Cheung PWH, Kawasaki S, et al. What determines immediate postoperative coronal balance and delayed global coronal balance after anterior spinal fusion for Lenke 5C curves? Eur Spine J 2021;30:2007-19. [Crossref] [PubMed]
- Liang J, Li S, Xu D, et al. Risk factors for predicting complications associated with growing rod surgery for early-onset scoliosis. Clin Neurol Neurosurg 2015;136:15-9. [Crossref] [PubMed]
- Sun ZJ, Qiu GX, Zhao Y, et al. Dual growing rod treatment in early onset scoliosis: the effect of repeated lengthening surgeries on thoracic growth and dimensions. Eur Spine J 2015;24:1434-40. [Crossref] [PubMed]
- Watanabe K, Uno K, Suzuki T, et al. Risk factors for complications associated with growing-rod surgery for early-onset scoliosis. Spine (Phila Pa 1976) 2013;38:E464-8. [Crossref] [PubMed]
- Jiang H, Hai JJ, Yin P, et al. Traditional growing rod for early-onset scoliosis in high-altitude regions: a retrospective study. J Orthop Surg Res 2021;16:483. [Crossref] [PubMed]
- Akbarnia BA, Pawelek JB, Hosseini P, et al. Treatment of Early-onset Scoliosis: Similar Outcomes Despite Different Etiologic Subtypes in Traditional Growing Rod Graduates. J Pediatr Orthop 2022;42:10-6. [Crossref] [PubMed]
- Hill G, Nagaraja S, Akbarnia BA, et al. Retrieval and clinical analysis of distraction-based dual growing rod constructs for early-onset scoliosis. Spine J 2017;17:1506-18. [Crossref] [PubMed]
(English Language Editors: J. Reylonds)



