
Denosumab in pediatric bone disorders and the role of RANKL blockade: a narrative review
Introduction
Denosumab, a monoclonal antibody against receptor activator for nuclear factor kappa B ligand (RANKL), has recently seen widespread use in the treatment of adult osteoporosis and the prevention of bone-related events of bone metastases due to its ability to reduce bone turnover, increase bone density, and prevent the progression of certain bone neoplasms (1-4). However, the pharmacokinetics and pharmacodynamics of denosumab in children are not yet clearly known, and denosumab therapy in children is not currently licensed. Despite the scarcity of drugs available for refractory pediatric orthopedic-related diseases, including osteogenesis imperfecta (OI), fibrous dysplasia (FD), aneurysmal bone cyst (ABC), and secondary osteoporosis, there are primarily literature reports of clinicians successfully treating skeletal system—immature children with denosumab, although these children also experienced some side effects that are rare in adults (5-8). These adverse effects included hypocalcemia, increased risk of infection when denosumab was applied in combination with steroids, and a serious adverse effect reported in adults—medication-related osteonecrosis of the jaw (MRONJ). To date, the effectiveness and safety of denosumab therapy in the field of pediatric orthopedic-related diseases is still under debate, particularly regarding standardized clinical practices and the prevention of bone turnover rebound after discontinuation in children (9-11). The aim of this review is thus to provide a historical perspective on applying denosumab in the management of refractory pediatric orthopedic diseases using the available data to encourage best practices. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-276/rc).
Methods
Literature to build this review was searched for and retrieved from the PubMed, EMBASE, and Cochrane Library databases. The specific literature search strategy is presented in Table 1. And literature search queries on PubMed was listed in Table S1.
Table 1
| Item | Specification |
|---|---|
| Date of search | December 3, 2021 |
| Databases and other sources searched | PubMed, Embase, and Cochrane Library were searched. Important missed citations in the literature were searched for manually |
| Search terms used | Denosumab, child, adolescent, pediatric, giant cell tumor of bone, osteogenesis imperfecta, fibrous dysplasia, aneurysmal bone cyst |
| Timeframe | From the establishment of database to December 3, 2021 |
| Inclusion and exclusion criteria | Inclusion criteria: studies of applying denosumab in children younger than 18 years old; in the English language; and including case and series reports, retrospective cohort studies, and prospective studies |
| Exclusion criteria: lack of a detailed description of how to use denosumab and no data on effects or complications | |
| Selection process | Daoxi Wang and Qianyu Shi conducted the selection independently and then worked cooperatively in the literature screening. When there was disagreement on a piece of literature, third experts (Tao Ji or Xueyang Tang) was consulted |
Irrelevant studies were excluded through reading titles and abstracts. Those studies that recruited children who were under 18 years of age using denosumab for orthopedic disease were included, and those with unclear denosumab administration or missing treatment and follow-up data were excluded. The literature ultimately included in the review is listed in Tables 2-5.
Table 2
| Study | Year | Patient age | Dose and interval of denosumab | Duration of treatment | Effect of treatment | Adverse events during treatment | Adverse events after treatment |
|---|---|---|---|---|---|---|---|
| Semler et al. (6) | 2012 | 4 boys age 5–18 years (type 6) | 1 mg/kg every 8–12 weeks | 24 months | Increased BMD, improved pain | Mild hypocalcemia (1 patient) | Unknown |
| Hoyer-Kuhn et al. (12) | 2014 | 4 boys age 5–18 years (type 6) | 1 mg/kg every 8–12 weeks | 24 months | Increased BMD, improved pain | Mild hypocalcemia (1 patient) | Unknown |
| Ward et al. (13) | 2016 | 1 child age 2 years (type 6) | 1 mg/kg every 4–12 weeks | 12 months | Increased BMD, improved mobility, more osteoclasts formed | Severe hypercalcemia | None |
| Hoyer-Kuhn et al. (14) | 2016 | 10 children age 5–11 years (types 1, 3, and 4) | 1 mg/kg every 12 weeks | 12 months | Increased BMD and increased height; bone pain did not change | Mild hypocalcemia (1 patient) | Mild hypercalcemia |
| Uehara et al. (15) | 2017 | 1 child age 14 years | Every 6 months | 24 months | Increased BMD | None | Unknown |
| Trejo et al. (16) | 2018 | 4 children age 1.9–9 years (type 6) | 1 mg per kg every 3 months | Mean 24 months | Increased BMD | Mild hypercalcemia and persistent hypercalciuria, nephrocalcinosis, rapid bone loss | None |
| Hoyer-Kuhn et al. (17) | 2019 | 10 children, mean age 8.6 (6.16–12.13 years) (types 1, 3, and 4) | 1 mg per kg every 20.3 weeks (depending on the individual urinary excretion course of deoxypyridinoline) | 53.04 weeks (± SD 6.30) | Increased BMD | Arthralgia, muscle pain, symptomatic hypercalciuria | Symptomatic hypercalciuria |
SD, standard deviation; BMD, bone mineral density.
Table 3
| Study | Year | Patient age | Dose and interval of Denosumab | Duration of treatment | Effect of treatment | Adverse events during treatment | Adverse events after treatment |
|---|---|---|---|---|---|---|---|
| Boyce et al. (7) | 2012 | 9-year-old boy | 1–1.5 mg/kg monthly | 7 months | Decreased tumor expansion and pain | Mild hypophosphatemia | Severe hypercalcemia |
| Wang et al. (18) | 2014 | 9-year-old boy | 1–1.5 mg/kg monthly | 7 months | Decreased tumor expansion and pain | Mild hypophosphatemia | Severe hypercalcemia |
| Majoor et al. (19) | 2019 | Median 8.8 years | Subcutaneous denosumab 60 mg at 3- or 6-month intervals | 15.5 (range, 12–19) months | Reduction in bone pain | None | None |
| Raborn et al. (20) | 2021 | 13-year-old girl | 1 mg/kg every 4 weeks | 3.5 years | Resolution of pain and progressive increase in extracranial bone density | None | Hypercalcemia, relapse |
Table 4
| Study | Year | Patient’s age | Dose and interval of denosumab | Duration of treatment | Effect of treatment | Adverse events during treatment | Adverse events after treatment |
|---|---|---|---|---|---|---|---|
| Lange et al. (21) | 2013 | 8- and 11-year-old boys | 70 mg/m2 monthly | Ongoing | Cyst partial regression, improved pain | Mild hypocalcemia (1 patient) | Unknown |
| Pelle et al. (22) | 2014 | 5-year-old boy | 1.2–1.6 mg/kg monthly | 12 months | Cyst regression, healed fracture, improved pain | None | Unknown |
| Fontenot et al. (23) | 2018 | 13-year-old girl | Subcutaneous denosumab (120 mg) given every 4 weeks (with additional 120 mg SC doses on days 8 and 15 in cycle 1 only) | 12 months | Pain improved, decreased tumor size | None | None |
| Raux et al. (24) | 2019 | Median age was 8 years (range, 7–17 years) | 70 mg/m2 | A median of 12 months (range, 4–23 months) | Free of pain, and the neurological deficits in 3 patients had improve | Hypocalcemia | Hypercalcemia |
| Harcus et al. (25) | 2020 | 1 child, 13 years old | Subcutaneous denosumab (70 mg/m2) on a weekly–2 months–3 months–4 months–6 months | Pain free, new bone formation in the lesion | Calcification of the lower limb growth plates | Rebound hypercalcemia | |
| Fadavi et al. (26) | 2021 | 1 child, 13 years old | 120 mg every 4 weeks | 12 months | Neurologic symptoms fully recovered | None | None |
Table 5
| Study | Year | Patient age | Dose and interval of denosumab | Duration of treatment | Effect of treatment | Adverse events during treatment | Adverse events after treatment |
|---|---|---|---|---|---|---|---|
| Chawla et al. (27) | 2013 | 10 children ≥12 years old | 120 mg monthly | Ongoing | Decreased tumor size, improved pain | None | Unknown |
| Karras et al. (28) | 2013 | 10-year-old girl | 120 mg monthly | 24 months | Decreased tumor size, improved pain | Mild hypocalcemia and hypophosphatemia | Severe hypercalcemia |
| Gossai et al. (29) | 2015 | 10-year-old girl | 120 mg monthly | 24 months | Decreased tumor size, improved pain | Mild hypocalcemia and hypophosphatemia | Severe hypercalcemia |
| Setsu et al. (30) | 2016 | 10-year-old boy | 120 mg monthly | 14 months | Decreased tumor size, improved pain | None | Severe hypercalcemia |
| Kobayashi et al. (31) | 2015 | 10-year-old boy | 120 mg monthly | 14 months | Decreased tumor size, improved pain | None | Severe hypercalcemia |
| Uday et al. (9) | 2018 | 14-year-old girl and 15-year-old boy | 120 mg subcutaneously on days 1, 8, 15, and 28, and then every 4 weeks | 1.3 years | Unknown | Unknown | Osteonecrosis of the jaw rebounded hypercalcemia, acute kidney injury |
| Sydlik et al. (32) | 2020 | 6–17 years old | 60 mg on days 1, 8, 15, and 28 and then once a month | 7–17 months | Decreased tumor size | Unknown | Tumor relapse, hypercalcemia |
| Reddy et al. (33) | 2021 | 14-year-old and 16-year-old | 120 mg subcutaneously once weekly for 3 weeks during the first cycle then once every 4 weeks with plans to complete a total of 26 cycles | 14/26 (26 months) | Reduction in tumor size | Mild hypocalcemia | None |
Discussion
RANK/RANKL/osteoprotegerin (OPG) signaling pathway, childhood bone metabolism, and the history of denosumab
The human skeleton is an organ with a dynamic metabolism that is continuously remodeled throughout life to recover from minor bone fractures and to maintain mineral homeostasis in response to diverse environmental stimuli. Remodeling is a microscopic process achieved through the coordinated action of osteoclasts and osteoblasts. Old bone resorption in osteoclasts and new bone formation in osteoblasts are tightly coupled in time and space (34,35).
The growth of bone in children is a strictly controlled process of breaking bone remodeling homeostasis conditions. Linear bone growth occurs when bone formation exceeds bone resorption at the epiphyseal growth plate due to local bone mass growth. Bone thickening and enlargement occur in the remodeling process of the periosteum and bone marrow cavity (36,37). The vigorous osteogenesis process below the periosteum leads to an increase in bone mass and bone thickening, while the bone dissolution process in the bone marrow cavity leads to an increase in bone absorption and bone marrow cavity enlargement. As a result, the bone thickens, the marrow cavity enlarges, and the bone mass increases.
The RANK/RANKL/OPG signaling pathway is an important bone metabolic pathway that has been widely studied over the past 20 years (38,39). RANKL is a transmembrane protein expressed on the surface of bone tissue cells and T lymphocytes and is involved in the mature differentiation of osteoclasts. RANK, which is highly expressed in osteoblasts and osteocytes, is the receptor protein of RANKL. When RANK and RANKL combine, osteoclast activation and differentiation are induced, promoting bone absorption and bone turnover, decreasing bone mineral density (BMD), and potentially even inducing osteolytic lesions. OPG is a transmembrane protein produced by bone marrow stromal cells that can bind to the RANKL-RANK complex to inhibit the activity of osteoclasts. RANK/RANKL/OPG are the centers of the hub and enable the skeleton to establish and maintain bone strength (Figure 1).
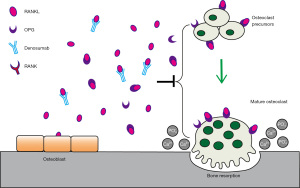
Denosumab is a subcutaneously administered, wholly human-derived, highly specific monoclonal antibody against RANKL. It binds RANKL with high affinity and specificity, initiating a metabolic process similar to that induced by OPG that inhibits osteoclast activation and differentiation, reduces bone resorption, and indirectly increases BMD (40). Denosumab was approved by the U.S. Food and Drug Administration (FDA) in 2010 as Prolia, initially listed alongside bisphosphonates as a first-line treatment for postmenopausal osteoporosis. Denosumab was launched in November 2010 under the name Xgeva for the treatment of skeletal problems in patients with solid tumors with bone metastases. In 2013, the FDA approved denosumab for the treatment of adult and skeletally mature adolescent patients with giant cell tumors of the bone (GCTBs) that cannot be resected or for which resections would result in significant morbidity. Indications were also extended to multiple myeloma and hypercalcemia just a few years later (41,42). A growing amount of literature supports the efficacy of denosumab as an inhibitor of bone resorption, with reports suggesting that common side effects in adults include hypocalcemia, muscle and joint pain in the extremities, rare cases of osteonecrosis of the jaw, and atypical femoral fractures (43-45).
Denosumab is a far more potent inhibitor of bone resorption than are bisphosphonates, which work by binding to hydroxyapatite and inhibiting osteoclast activity (46). Denosumab has an advantage over bisphosphonates, exhibiting better compliance, comfort, and ease of use by patients and requiring only subcutaneous injection. Denosumab therapy was generally well tolerated in multiple large trials, with no significant increase in adverse event incidence compared with that with placebo or bisphosphonates (47). However, because denosumab inhibits bone turnover with a shorter half-life than do bisphosphonates, the effect is completely reversible after withdrawal.
Thus far, the effects of denosumab on the growing skeleton have not been well described, the pharmacokinetics and pharmacodynamics of denosumab in children are not yet fully understood, and there is no consensus for standardized clinical practices in children. Moreover, scattered reports have revealed mixed results on the use of denosumab for various conditions in children (Figure 2). Controversies regarding denosumab use in children mainly concern its safety, specifically the optimal dose in individual children, dose frequency, therapy duration, impact on children’s epiphyseal development, and the means to preventing post-discontinuation rebound (6,12).
OI
OI was the first disease for which an attempt to treat pediatric patients with denosumab was reported, as OI is the most common primary osteoporosis in children. Patients with OI are routinely treated with bisphosphonates, but type 6 OI has a poor therapeutic response to bisphosphonates. Type 6 OI is an autosomal recessive form caused by the SERPINF1 (Serpin family F member 1) mutation. There is experimental evidence suggesting that functional deficiency in SERPINF1 activates osteoclasts through the RANK/RANKL pathway (48). Based on preliminary study and due to the lack of available drugs for use in children, denosumab was first used by Semler et al. to treat 4 children with type 6 OI whose disease was refractory to bisphosphonates (6). The approach they used was to inject denosumab subcutaneously every 3 months (1 mg/kg BW) with an initial 12-week interval, and no serious side effects were found. A rapid decline in bone resorption was detected after each denosumab dose, and bone resorption returned to pretreatment levels at approximately 6 weeks, so the dose interval was adjusted to approximately 10 weeks. During the treatment period, no serious uncomfortable reactions were observed in the 4 children, improved osteoporosis and increased BMD became apparent, and only 1 instance of asymptomatic hypocalcemia was noted.
In another report, a boy with type 6 OI was treated with denosumab (1 mg/kg every 3 months) and received conventional calcium supplements during treatment. No hypercalcemia or hypocalcemia occurred. However, persistent bone fractures were found after 12 months of initial treatment. After that, subsequent denosumab treatment led to a rise in BMD. Interestingly, 2 biopsies in the denosumab treatment period demonstrated an increase in the number of osteoclasts compared to the pretreatment level (13).
After encouraging preliminary data were reported, additional studies have been conducted on the treatment of OI in children. In 2016, a prospective study involving 10 children with OI treated with denosumab was published. The authors evaluated the safety and efficacy of denosumab for the treatment of different types of OI by which denosumab’s dose interval varied from 3 to 12 months (1 mg/kg BW) (14). All the children studied could safely tolerate denosumab therapy; only 1 child with asymptomatic hypocalcemia was observed during the therapy period, and coincidentally, mild asymptomatic hypercalcemia was observed in several patients prior to the fourth dose of denosumab. Uehara et al. reported a similar result in their 2017 study (15). These trials confirmed the efficacy of denosumab in OI, with an increase in bone BMD observed during treatment, although pain symptoms and activity did not improve. These findings also raise further questions, and the optimal dose and appropriate dose interval of denosumab based on the metabolic characteristics of children warrant further research and confirmation.
In 2018, Trejo et al. evaluated the use of denosumab in 4 children with type 6 OI using the most classical treatment strategy (1 mg per kg body mass every 3 months) (16). Hypercalcemia occurred in all 4 children during the treatment period. In 2 children aged 3.9 and 4.6 years, respectively, hypercalcemia occurred between 7 and 12 weeks after the most recent injection of denosumab. BMD declines rapidly when the dose interval is increased up to 6 months for clinical reasons. Another study found that the inhibition of absorption by denosumab appeared to last only 6 to 8 weeks, so shortening the dose interval from the initial 12 weeks to a minimum 10-week interval may prevent hypercalcemia (12). However, 1 patient in this study developed hypercalcemia only 7 weeks after injection of denosumab. The authors raised two critical issues: whether the denosumab dose interval should be shorter in children than in adults and whether denosumab along with bisphosphonates can be used to treat OI to prevent discontinuation turnover rebound (16).
A recent study in 2019 on denosumab for OI in children sought to determine the optimal dose and interval for denosumab in children to address questions raised by previous authors. The investigators administered denosumab at a dose of 1 mg/kg body weight to 10 pediatric patients with OI and, for the first time, used urinary deoxypyridinoline (DPD) levels as a marker to assess osteoclastic activity and to personalize the interval of denosumab injection. When the level of DPD/creatinine (CREA) prior to injection of denosumab was increased, a second dose of denosumab was administered (17). All 10 children tolerated denosumab therapy, and only a few patients reported muscle and joint pain during the treatment period, with these cases all being relieved without intervention. In their study, symptomatic hypercalciuria was observed 12 months after drug withdrawal, and renal function was assessed and found not to be impaired. Finally, the denosumab interval was delayed from 12 to 20.3 weeks. The increase in bone mass and BMD during the trial was significantly higher during the follow-up period, proving its efficacy in treating OI. Constant bone growth assessment throughout the 2-year observation period showed that bone growth in pediatric patients was not affected. This study demonstrates the possibility and necessity of individualized treatment strategies based on urinary bone resorption marker levels.
The above studies are summarized in Table 2 and indicate that denosumab might represent a valid alternative approach to treating OI in children. Further studies need to be carried out to determine the safety and standardized clinical practices of denosumab in children. The level of DPD/CREA is an example of good practices for monitoring medication safety.
FD and McCune-Albright syndrome (MAS)
FD is a rare disorder of the skeletal system caused by mutations in the alpha subunit of the Gs stimulator protein (Gsα), which activates the cyclic adenosine phosphate (cAMP) regulatory protein. Because normal bone and marrow are replaced by fibrous bone tissue, clinical manifestations vary widely and can include dysfunction, deformity, and pain. FD may be associated with skin hyperpigmentation and endocrine hyperfunction, including hyperthyroidism, precocious puberty, excess growth hormone, and Cushing syndrome. FD combined with one or more extraosseous manifestations is defined as MAS (49).
Because of the wide range of FD lesions, it is difficult to completely cure the disease. At present, the treatment goal for FD is mainly to control disease progression and prevent bone and severe osteoarticular deformities. Surgical treatment is performed only in cases of acute bone fracture or severe osteoarticular malformations. At present, the mainstream treatment drug is bisphosphonates, which can effectively relieve the pain caused by FD; however, preliminary evidence indicates that they cannot alleviate the progression of the disease (50,51).
It has been speculate that bisphosphonates’ lack of effect in this regard is due to its action requiring the binding to the mineralized matrix, which is greatly reduced in FD tissues (19,52). Recent research has revealed that skeletal neoplasm development depends on RANKL and cAMP/protein kinase A. A growing number of studies have reported that the inhibition of RANKL by denosumab is effective against GCTB, and a preliminary study of FD and ABC demonstrated that they shared a similar pathogenic lesion with GCTB.
Based upon these data and research, denosumab was first used to treat FD in a child with a rapidly expanding FD lesion by Boyce et al. in 2012 (7). They applied denosumab in a 9-year-old boy who was diagnosed with MAS due to extensive skeletal FD, hyperthyroidism, and skin pigmentation abnormalities. He was first treated with bisphosphonates for 1 year, but the pain was not relieved, and the lesion continued to progress. After denosumab (1–1.5 mg/kg every 1 month) was injected for 7 months, the rate of progression slowed, and the pain improved. However, acute rebound hypercalcemia occurred 2 months after drug withdrawal and was relieved by treatment with bisphosphonates and calcitonin.
After the first satisfactory result of treatment of a child with FD was reported, additional groups reported their experience of using denosumab in children with FD. In the studies of Wang et al. (18), Majoor et al., (19) and Raborn et al. (20), the safety and efficacy of denosumab in children with FD exhibited good results. These studies confirmed the effectiveness of denosumab in the treatment of FD, in alleviating disease progression, and in relieving pain. No serious adverse events were observed during the denosumab therapy period. Both Wang et al. and Raborn et al. reported there to be severe hypercalcemia after drug withdrawal, while Raborn et al. noticed a tendency of tumor relapse after drug withdrawal. Another unexpected benefit was reported by Wang et al., who found that denosumab treatment seemed to not affect the growth or development of the epiphyseal plates in the children.
A recent study on a mouse model of FD revealed that treatment with an anti-RANKL antibody prevented the formation of new lesions and promoted skeletal stem cell differentiation into functional osteoblasts, resulting in mineralized lamellar bone formation (50). This result showed that compared with bisphosphonates, which require binding to the mineralized matrix to impact osteoclasts, denosumab is a promising treatment because it does not require matrix incorporation and can directly target ectopic osteoclasts.
Data on children with FD reported in the literature using denosumab are summarized in Table 3. The FD patient follow-up in these data is relatively short, and the longest period with follow-up data was 3.5 years. Most treated children were still in an immature skeleton condition. Consequently, the long-term safety and efficacy of FD treatment are still unknown. Furthermore, since effective management of refractory FD requires long-term treatment, it is unclear whether continuous denosumab treatment will affect the growth and development of children’s epiphyses. To reduce the effect of long-term denosumab on bone development, intermittent medication may work, but intermittent treatment leads to the problem of rebound bone turnover and an increased risk of neoplasm relapse. To solve these problems, prospective trials of denosumab in the treatment of FD in children and its withdrawal rebound trial (NCT03571191) are underway.
Aneurysmal bone cyst
ABC, which accounts for approximately 5% of benign bone tumors, is a locally destructive benign bone tumor, with 90% of cases occurring in young people under the age of 20 years and 25% of these being secondary ABCs that may form within preexisting benign or malignant bone tumors, including GCTB and FD (53,54). Recent studies have found translocation or gene fusions of the USP6 gene on chromosome 17p.3 in 75% of primary ABCs, pointing to the true oncological etiology and representing the molecular confirmation ABC (55,56).
The most common body location of ABC is the long diaphysis and posterior spine, but other sites may also be involved. The chief complaint is pain and swelling in the affected area, and pathological fractures may sometimes be observed. A relatively well-defined lytic “dilatative” lesion can be seen on plain radiographs and may rupture from the periosteum and have a soapy appearance (57,58).
The mainstream treatment for ABC is curettage with various local adjuvants, such as application of a high-speed burr, argon beam, phenol, etc. The incorporation of new adjuvants in treatment has significantly reduced the recurrence rates (7–12.5%) compared to those with curettage alone (59%), with intralesional doxycycline being particularly effective (59). ABC accounts for 15% of primary spinal tumors, of which 20% to 30% occur in the sacrum, and surgical resection carries high risk of life-threatening bleeding and damage to nerves or adjacent visceral organs and leads to disability and relapse. Treatment of ABCs in their critical locations remains a tremendous challenge (60).
A previous study revealed that the pathogenesis of ABC is similar to that of GCTB. The activation of RANK-RANKL signaling is crucial to the development and progression of ABC, and tumor spindle cells in ABC express high levels of RANKL, leading to osteoclast-like giant cell activation and osteolysis (22). Due to the histopathological similarities between GCTB and ABC, off-label denosumab therapy has been used in children with ABC.
Alhumaid et al. summarized the most current clinical experiences of denosumab-treated ABC and found that denosumab therapy could offer therapeutic clinical and radiological benefits in patients with ABC, particularly those patients with locally advanced, recurrent, or inoperable disease. Although the study was mainly limited to adults, it still can serve as a useful reference and guide for the treatment of children (61).
In 2013, Lange et al. were the first to apply denosumab in children with ABC (21). The 2 treated children had recurrent and refractory ABC lesions in the fifth cervical vertebra. Considering the special position, high potential for side effects, and risks of various treatment methods, Lange et al. finally chose individualized denosumab for trial treatment. The efficacy of denosumab in children with ABC was demonstrated and yielded good clinical results with improved pain and radiological short-term response with partial regression of the cyst. During the treatment period, only 1 child reported mild hypocalcemia, and no other serious adverse reactions occurred. Because the drug continues to be used, the adverse effects after discontinuation are unknown.
In 2014, Pelle et al. reported a 5-year-old male patient with a large and locally aggressive ABC involving the sacrum. The patient received 10 doses of denosumab, and symptoms were relieved, with complete remission of lower genitourinary symptoms and no radiological signs of recurrence (22).
In 2018, Fontenot et al. reported a 13-year-old female patient with a history of recurrent ABC involving the distal fibula (long bone). The patient received preoperative denosumab for 1 year (120 mg given every 4 weeks with additional 120-mg subcutaneous doses on days 8 and 15 in cycle 1) followed by intralesional curettage with high-speed burring and cement augmentation (23). In 2019, Raux et al. reported 5 cases of children with ABC, with an average age of 8 years, which was the highest number of cases reported in children thus far (24). The main lesion positions were the lumbosacral vertebrae and femoral neck. Denosumab was administered to treat these inoperable or refractory ABCs provided clinical and radiological improvements, with further tumor progression after denosumab discontinuation occurring in only 1 of the 5 patients. Hypocalcemia occurred in 2 patients, whereas hypercalcemia occurred in 2 patients. These data suggest a possible elevated risk of rebound hypercalcemia when denosumab therapy is prolonged. Dunnion et al. reported dense sclerotic metaphyseal bands caused by denosumab therapy in a 12-year-old girl with ABC, indicating the need to be aware of its alarming effects on the growing skeleton (62). Fadavi et al. reported a challenging case, a 13-year-old boy with cervical ABC progression to quadriparesis. He experienced significant regression after the first dose of denosumab, and after 12 courses, neurological symptoms recovered completely (26).
The efficacy of denosumab in ABC therapy has been confirmed by the above-mentioned literature (the data are shown in Table 4). However, different pathological situations, different treatment strategies and dose intervals, and various performance side effects were demonstrated. Adverse reactions include gastrointestinal discomfort, hypocalcemia, and calcification of the lower limb growth plates. Hypercalcemia after drug withdrawal was reported in 4 studies. The dosage varied from 1.2–1.6 mg/kg to 70 and 120 mg, with intervals including 1 week, 2 weeks, 4 weeks, and 6 months. Therefore, further information is needed to determine the optimal dose, dose interval, and therapy duration in children who are skeletally immature. The literature also suggests that appropriate monitoring is required once denosumab use has been initiated and during the drug withdrawal process. It is recommended that blood calcium concentration is monitored every 2 months and that parents are educated on the common clinical manifestations of hypercalcemia to detect and prevent adverse reactions in advance (25).
Giant cell tumor of bone (GCTB)
GCTB is a locally invasive osteoclast stromal tumor. At present, the challenge in the treatment of GCTB is to reduce the recurrence rate, and it is critically necessary to find an effective adjuvant therapy for refractory GCTB when it is located in particularly difficult sites, such as the spine, pelvis, or facial bones, when resection could result in severe morbidity (63-65).
Denosumab is approved by the US Food and Drug Administration (FDA) for treatment of patients whose GCTB cannot be surgically removed. Denosumab can be used in adults and bone-matured adolescents, while a growing number of studies support denosumab as a valid therapeutic approach for GCTB in adults and skeletally mature adolescents (27-29,32,66).
When denosumab is used to treat GCTB, tumor recurrence after drug withdrawal can be disappointing. Research data indicate there to be a regression of the therapeutic effect, and up to 26% of individuals exhibit disease progression after withdrawal. Basic studies have shown that denosumab mainly acts on osteoclastic megakaryocytes in bone tissue but has no effect on mesenchymal cell proliferation and cannot completely prevent the progression of the disease. Other alternative strategies and new therapies need to be studied concerning this mechanism (67,68).
The incidence of GCTB is low in children. Primarily, studies have reported that in GCTB occurring in children before epiphyseal maturity, there is a higher incidence of multicenter lesions of the vertebrae (27,69). There are limited drugs and strategies for treating refractory GCTB. The use of denosumab for GCTB therapy in children was first reported by Karras et al. in 2013 (28). The researchers administered denosumab to adolescents over the age of 12 at the recommended adult dose of 120 mg per month to halt the progression of lesions, and there were no reported serious adverse reactions.
Furthermore, other groups have reported the successful application of denosumab in the treatment of GCTB in children, including Gossai et al. (29), Kobayashi et al. (31), and Setsu et al. (30). Kobayashi et al. reported a case in whom sclerosing bands were present in almost all radiographs of the metaphysis. However, the patient showed no signs of growth retardation at the last follow-up.
In 2021, Reddy et al. reported their experience with denosumab in 2 children with GCTB and pulmonary metastasis, achieving satisfactory results (33). During the denosumab treatment period, a reduction in the size of the primary lesions and lung metastatic lesions was observed, and pain was relieved. However, both patients developed mild hypocalcemia.
All these data (in Table 5) support denosumab as an effective treatment in children which can reduce pain and alleviate lesion progression. In contrast to adults, children frequently develop acute rebound of bone metabolism with severe and life-threatening hypercalcemia several months after discontinuation, often requiring hospitalization and bisphosphonate treatment. This finding represents a serious safety issue that is rarely reported in adults and appears to be primarily associated with pediatric patients. It has been speculated that the basal bone metabolic rate of children is higher than that of skeletally mature adults. Bone metabolism tends to rebound rapidly after drug withdrawal, resulting in increased bone absorption and elevated blood calcium levels. Although Uday et al. reported the first case of rare jaw osteonecrosis in a young patient with GCTB treated with denosumab. If jaw symptoms occur the drug should be stopped (9).
Central giant cell granuloma (CGCG)
CGCG is a bony destruction with multinucleated giant cells, which usually occurs only in the mandible and rarely occurs in the maxilla. It usually grows slowly with local cortical expansion without pain symptoms. Sometimes it can demonstrate invasive lesions with rapid growth and pain. Its incidence rate is about 1.1 per million, with a higher prevalence in children and adolescents, as well as in females (70). The first-line treatment is surgical excision or curettage, which is associated with higher of dentition loss, mastication and cosmetic defects, sensory loss, and up to 70% recurrence (64). Therefore, for CGCG that is difficult to treat surgically with a good cosmetic result or that recurs after surgery, a drug-based comprehensive treatment regimen should be initiated.
In the early stages of treatment, there were some reports of denosumab therapy in adult patients with CGCG. After successful results were obtained in adults, reports of denosumab treatment in children gradually began to emerge (71-73).
In 2014, Naidu et al. were first to report a trial of denosumab in children with CGCG (8). In another retrospective study of denosumab treatment that followed up the child for 75 months, the treatment was found to be generally safe. However, the patient relapsed and developed rebound hypercalcemia 14 months after discontinuation (74).
The largest group of children studied with CGCG treated using denosumab was reported in Texas Children’s Hospital in 2021 (75,76). In this study, 6 children aged 5 to 12 years were followed up for an average of 20 months. After loading doses on days 1, 8, and 15 of cycle 1, patients received a dose of 70 mg/m2 (maximum 120 mg) every 4 weeks, and generally received 12 doses until treatment ended. Favorable response, symptom relief, lesion reduction, and improved BMD were observed in all denosumab-treated patients. Moreover, 4 patients developed mild hypocalcemia, and 3 patients developed rebound hypercalcemia and acute kidney injury, which occurred 5 months after drug withdrawal. No rebound hypercalcemia was observed in any child after drug reduction via descending dosage. In addition, the bone age for all patients remained normal.
Secondary osteoporosis and other rare diseases
Other cases reported in children include those with osteopetrosis, hypercalcemia (5,10), juvenile Paget disease (JPD) (77), and secondary osteoporosis in children (78,79). Secondary osteoporosis in children mainly includes disuse osteoporosis and glucocorticoid-related osteoporosis.
Shroff et al. reported that 2 children (3 and 12 years old) with functional-loss mutations in the TNFRSF11A gene encoding RANK underwent hematopoietic stem cell transplantation, resulting in strong osteoclast activation and refractory hypercalcemia. In both patients, denosumab led to rapid normalization of serum calcium levels (5). JPD is an abnormal and rare genetic disorder of rapid bone remodeling in infants or individuals in early childhood. Symptoms extend beyond the bone and can include hearing loss and retinopathy. Bisphosphonates were the first recommended drug. However, several clinical trials have confirmed that while bisphosphonates can relieve bone lesion progression in JPD, they cannot alleviate extraosseous progression, which may include hearing and vision impairment. Grasemann et al. reported an unexpected improvement in hearing test results in an 8-year-old girl with severe JPD treated with denosumab, in addition to the alleviation of bone damage. However, serious hypercalcemia occurred post-discontinuation in JPD, which suggests careful consideration is needed for the application of this treatment approach (77).
Anastasilakis et al. reported the treatment of children with severe primary osteoporosis with denosumab and indicated denosumab to be highly effective, with remarkable clinical and radiological responses. Transient hypercalcemia occurred but was probably due to amplification of the effect of growth spurts and puberty on bone remodeling by the transient, short-term discontinuation of the drug (80).
Common causes of secondary osteoporosis include immobility, inflammatory conditions treated with steroids, Duchenne muscular dystrophy (DMD) and other myopathies, leukemia, and other cancers. Huang et al.’s preliminary study found denosumab to be an effective treatment for low BMD in childhood cancer survivors, although hypocalcemia occurred in 40% of patients (81). Scheinberg et al. reported their experience in treating disuse osteoporosis caused by cerebral palsy with denosumab (78). Additionally, Kumaki et al. described the first experience of denosumab treatment for glucocorticoid-induced osteoporosis in a patient with DMD (82), with the BMD increasing to some extent.
Treatment strategy and safety of denosumab in children
The above-mentioned studies demonstrated that denosumab is a promising treatment for bone disorders in children and represents a valid alternative therapeutic approach to improve pediatric bone health. A summary of the studies reporting the use of denosumab in children and adolescents in is presented in Table 6.
Table 6
| Disease | Dose and interval of denosumab | Preparation before start denosumab | Supplements in therapy | Monitoring in therapy | Dose modifications | Monitoring after end of therapy |
|---|---|---|---|---|---|---|
| ABC | <45 kg 70 mg/m2 every month; ≥45 kg 120 mg every month | Treat and prevent oral diseases; correct hypocalcemia and vitamin D deficiency | Calcium 500 mg/day, vitamin D 400 IU/day | Serum calcium ≥2 mmol/L and ≤2.9 mmol/L | Hold denosumab when serum calcium <2 mmol/L or if denosumab-related grade 3 or 4 adverse events occur | Monitoring calcium >6 months, if symptomatic hypercalcemia is observed, decrease doses every 3 months |
| GCTB | ||||||
| CGCG | ||||||
| FD | 1–1.5 mg/kg every month | |||||
| OI | 1 mg/kg every 3 months |
ABC, aneurysmal bone cyst; GCTB, giant cell tumor of bone; CGCG, central giant cell granuloma; FD, fibrous dysplasia; OI, osteogenesis imperfecta.
To date, serious side effects, such as osteonecrosis of the jaw and asymptomatic femoral fracture, have rarely been reported in children. However, there are still several concerns about pediatric clinical use that deserve further discussion and investigation.
Denosumab is a powerful anti-resorption agent, and early preclinical studies in rodents and primates have shown that it can significantly inhibit bone growth and tooth eruption (83). Denosumab has a significant advantage over bisphosphonates due to the reversibility of its effect. Moreover, bisphosphonates need to bind to bone hydroxyapatite, which results in a longer half-life of bone, so their effect lasts longer. In the published data, denosumab has been shown to affect radiographic changes in the bone epiphysis, and while sclerotic epiphyseal bands may appear, they tend to fade with time (31,62). Although clinical data are limited, there are many reports of continued bone growth after denosumab use (18,84). Wang et al. examined the effects of denosumab treatment and discontinuation on skeletal growth plates in children. In a 9-year-old boy with FD who received denosumab for 7 months, imaging and histological analysis of the growth plates of the limbs showed that although sclerotic bands developed near the growth plate during the treatment, the sclerosing bands gradually dissolved after treatment, and the epiphyseal growth continued during and after treatment (18). Similar results were found in children treated with bisphosphonates, but there was no significant clinical significance for bone growth (18,85). Although limited by a small sample size, Wang et al. directly demonstrates that denosumab has no significant adverse effects on bone growth in children.
Previous case reports have mentioned the minor adverse reactions observed during the denosumab treatment period, including occasional asymptomatic hypocalcemia and hypophosphoremia, mild gastrointestinal reactions, self-relieving muscle and joint pain, and urinary calcium detection, which do negate its validity as a therapeutic approach. Safety concerns related to rebound bone turnover and hypercalcemia after discontinuation are currently the main concerns regarding the safe use of denosumab in children. Hypercalcemia after discontinuation of denosumab therapy in adults is rarely reported, but the incidence in children is significantly higher. Some authors speculate that the main cause of hypercalcemia in children is the basal bone metabolic rate being higher than that in adults (24,30). The stronger the inhibition of bone metabolism during treatment is, the higher the rebound after drug withdrawal.
High blood calcium levels could occur as early as 7 to 8 weeks after discontinuation, and some reported instances occurred 20 weeks after discontinuation. This kind of hypercalcemia can require hospitalization for pain control and/or hydration and correction of hypercalcemia; fortunately, patients can quickly recover through bisphosphonate treatment. A multifactorial analysis of thoracic vertebral fractures after drug discontinuation suggested bisphosphonates to be a protective factor against fracture after drug discontinuation. This finding provides a direction for denosumab discontinuation strategies, as discontinuation therapy with bisphosphonate can be routinely used to prevent hypercalcemia (32,86).
The pharmacodynamic effects of drugs in children are mainly affected by two factors: (I) levels of RANK/RANKL/OPG activity due to increased bone turnover related to the child’s bone growth rate and (II) body weight and surface area (87).
Therefore, the optimal denosumab dose and interval for children and how to regulate the duration of denosumab therapy through monitoring bone metabolic markers in blood or urine are the most urgent problems to be solved. Hoyer-Kuhn et al. (17) reported that by flexibly adjusting the injection interval of denosumab based on the content of the urinary metabolite DPD/CREA and extending the injection interval from 8 to 20 weeks, they could achieve the best efficacy results with no hypercalcemia, thus providing a good reference for the adjustment of denosumab administration. To prevent the occurrence of hypercalcemia, it is necessary to closely monitor the fluctuation of bone metabolic marker levels and maintain a stable state of bone metabolism in children requiring lifelong medication or young patients with a high metabolic rate in bone. Children who are about to cease denosumab therapy may need to reduce the dose gradually to achieve a stable balance of blood electrolytes. Therefore, the dose and interval of denosumab administration, the monitoring and adjustment strategy during drug use, the withdrawal strategy, and the monitoring strategy post-discontinuation need to be holistically considered.
In the short term, it is of great significance to strengthen the monitoring of markers of bone metabolism, strengthen the monitoring of blood marker cutoffs, and educate patients and their parents on the common symptoms of hypercalcemia for the early detection and prevention of hypercalcemia.
The main disadvantage of denosumab is that its effect on bone turnover and inhibitory effect on bone lesions are reversible. Some primary bone neoplasms will recur after discontinuation of denosumab therapy, which has been reported for GCTB. Therefore, long-term drug safety tests should be considered for patients who relapse after drug withdrawal. In addition, how to inhibit the continued proliferation of bone tumor stromal cells in bone neoplasms is another area worth further study.
Limitations and future directions
Some limitations to this review should be noted. Fist, all of the literatures gathered were comprised of case reports and retrospective studies, which lacked controlled research groups and prospective design, and the research samples were mixed with adults. The studies were greatly affected by various biasing factors. Therefore, the level of evidence-based evidence was not high, and any conclusions drawn from them should be carefully scrutinized. Additional clinical trial models are warranted regarding the optimal dose and discontinuation time of denosumab in children, and indeed, several are already underway. We look forward to the completion of valuable research in the future to better inform the safe use of denosumab in children.
Conclusions
Refractory benign bone lesions in children include a wide range of bone diseases; due to their unfavorable location and their inability to be surgically resected, they seriously affect the quality of life of children. Thus far, there is no approved effective and safe treatment strategy.
Denosumab, which has been approved for the treatment of bone disease in adults, has demonstrated a potential role in alleviating disease in multiple refractory bone lesions in children according to the published data.
Previous reports of denosumab in children showed that it was relatively safe, and no serious complications were observed. Hypocalcemia during the medication period could be prevented by calcium supplementation. Rebound hypercalcemia after discontinuation can be effectively controlled by close monitoring and bisphosphonate intervention. Again, no adverse effects on bone growth or development have been observed with denosumab therapy in children. Although it is currently used off-label, ongoing high-quality pediatric denosumab studies may achieve further advances.
Finally, denosumab needs to be used with caution in children with refractory benign bone lesions. A comprehensive assessment should be initiated before its use, including monitoring the development status of bones and teeth. During the therapy period, close monitoring of bone turnover markers to prevent safety issues related to bone turnover rebound and mineral homeostasis is necessary.
Acknowledgments
Funding: This study received funding from the National Natural Science Foundation of China (No. 81872180).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-276/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-276/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-276/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheng C, Wentworth K, Shoback DM. New Frontiers in Osteoporosis Therapy. Annu Rev Med 2020;71:277-88. [Crossref] [PubMed]
- Bonnet N, Bourgoin L, Biver E, et al. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest 2019;129:3214-23. [Crossref] [PubMed]
- McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821-31. [Crossref] [PubMed]
- Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 2012;18:4415-24. [Crossref] [PubMed]
- Shroff R, Beringer O, Rao K, et al. Denosumab for post-transplantation hypercalcemia in osteopetrosis. N Engl J Med 2012;367:1766-7. [Crossref] [PubMed]
- Semler O, Netzer C, Hoyer-Kuhn H, et al. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact 2012;12:183-8. [PubMed]
- Boyce AM, Chong WH, Yao J, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res 2012;27:1462-70. [Crossref] [PubMed]
- Naidu A, Malmquist MP, Denham CA, et al. Management of central giant cell granuloma with subcutaneous denosumab therapy. J Oral Maxillofac Surg 2014;72:2469-84. [Crossref] [PubMed]
- Uday S, Gaston CL, Rogers L, et al. Osteonecrosis of the Jaw and Rebound Hypercalcemia in Young People Treated With Denosumab for Giant Cell Tumor of Bone. J Clin Endocrinol Metab 2018;103:596-603. [Crossref] [PubMed]
- Mamedova E, Kolodkina A, Vasilyev EV, et al. Successful Use of Denosumab for Life-Threatening Hypercalcemia in a Pediatric Patient with Primary Hyperparathyroidism. Horm Res Paediatr 2020;93:272-8. [Crossref] [PubMed]
- Deodati A, Fintini D, Levtchenko E, et al. Mechanisms of acute hypercalcemia in pediatric patients following the interruption of Denosumab. J Endocrinol Invest 2022;45:159-66. [Crossref] [PubMed]
- Hoyer-Kuhn H, Netzer C, Koerber F, et al. Two years' experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis 2014;9:145. [Crossref] [PubMed]
- Ward L, Bardai G, Moffatt P, et al. Osteogenesis Imperfecta Type VI in Individuals from Northern Canada. Calcif Tissue Int 2016;98:566-72. [Crossref] [PubMed]
- Hoyer-Kuhn H, Franklin J, Allo G, et al. Safety and efficacy of denosumab in children with osteogenesis imperfect--a first prospective trial. J Musculoskelet Neuronal Interact 2016;16:24-32. [PubMed]
- Uehara M, Nakamura Y, Takahashi J, et al. Efficacy of Denosumab for Osteoporosis in Three Female Patients with Osteogenesis Imperfecta. Tohoku J Exp Med 2017;242:115-20. [Crossref] [PubMed]
- Trejo P, Rauch F, Ward L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact 2018;18:76-80. [PubMed]
- Hoyer-Kuhn H, Rehberg M, Netzer C, et al. Individualized treatment with denosumab in children with osteogenesis imperfecta - follow up of a trial cohort. Orphanet J Rare Dis 2019;14:219. [Crossref] [PubMed]
- Wang HD, Boyce AM, Tsai JY, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab 2014;99:891-7. [Crossref] [PubMed]
- Majoor BCJ, Papapoulos SE, Dijkstra PDS, et al. Denosumab in Patients With Fibrous Dysplasia Previously Treated With Bisphosphonates. J Clin Endocrinol Metab 2019;104:6069-78. [Crossref] [PubMed]
- Raborn LN, Burke AB, Ebb DH, et al. Denosumab for craniofacial fibrous dysplasia: duration of efficacy and post-treatment effects. Osteoporos Int 2021;32:1889-93. [Crossref] [PubMed]
- Lange T, Stehling C, Fröhlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J 2013;22:1417-22. [Crossref] [PubMed]
- Pelle DW, Ringler JW, Peacock JD, et al. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res 2014;164:139-48. [Crossref] [PubMed]
- Fontenot PB, Jesurajan J, Bui M, et al. Recurrent Aneurysmal Bone Cyst of the Distal Fibula Treated with Denosumab and Curettage. Case Rep Oncol Med 2018;2018:1574343. [Crossref] [PubMed]
- Raux S, Bouhamama A, Gaspar N, et al. Denosumab for treating aneurysmal bone cysts in children. Orthop Traumatol Surg Res 2019;105:1181-5. [Crossref] [PubMed]
- Harcus M, Aldridge S, Abudu A, et al. The Efficacy of Denosumab in the Management of a Tibial Paediatric Aneurysmal Bone Cyst Compromised by Rebound Hypercalcaemia. Case Rep Pediatr 2020;2020:8854441. [Crossref] [PubMed]
- Fadavi P, Arefpour AM, Hariri R, et al. Dramatic response of aneurysmal bone cyst to denosumab: Case report and literature review. Clin Case Rep 2021;9:e04993. [Crossref] [PubMed]
- Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013;14:901-8. [Crossref] [PubMed]
- Karras NA, Polgreen LE, Ogilvie C, et al. Denosumab treatment of metastatic giant-cell tumor of bone in a 10-year-old girl. J Clin Oncol 2013;31:e200-2. [Crossref] [PubMed]
- Gossai N, Hilgers MV, Polgreen LE, et al. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer 2015;62:1078-80. [Crossref] [PubMed]
- Setsu N, Kobayashi E, Asano N, et al. Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab 2016;34:118-22. [Crossref] [PubMed]
- Kobayashi E, Setsu N. Osteosclerosis induced by denosumab. Lancet 2015;385:539. [Crossref] [PubMed]
- Sydlik C, Dürr HR, Pozza SB, et al. Hypercalcaemia after treatment with denosumab in children: bisphosphonates as an option for therapy and prevention? World J Pediatr 2020;16:520-7. [Crossref] [PubMed]
- Reddy K, Ramirez L, Kukreja K, et al. Response to Denosumab in 2 Children With Recurrent Giant Cell Tumor of the Bone With Pulmonary Metastasis. J Pediatr Hematol Oncol 2021;43:e215-8. [Crossref] [PubMed]
- McDonald MM, Khoo WH, Ng PY, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021;184:1940. [Crossref] [PubMed]
- Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 1965;206:489-90. [Crossref] [PubMed]
- Rønne MS, Heidemann M, Schou A, et al. Tracking of bone mass from childhood to puberty: a 7-year follow-up. The CHAMPS study DK. Osteoporos Int 2018;29:1843-52. [Crossref] [PubMed]
- Gosman JH, Stout SD, Larsen CS. Skeletal biology over the life span: a view from the surfaces. Am J Phys Anthropol 2011;146:86-98. [Crossref] [PubMed]
- Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004;292:490-5. [Crossref] [PubMed]
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165-76. [Crossref] [PubMed]
- Sutjandra L, Rodriguez RD, Doshi S, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet 2011;50:793-807. [Crossref] [PubMed]
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082-92. [Crossref] [PubMed]
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol 2014;7:2113-22. [PubMed]
- Boyce AM. Denosumab: an Emerging Therapy in Pediatric Bone Disorders. Curr Osteoporos Rep 2017;15:283-92. [Crossref] [PubMed]
- Body JJ, Lipton A, Gralow J, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res 2010;25:440-6. [Crossref] [PubMed]
- Koldkjær Sølling AS, Harsløf T, Kaal A, et al. Hypercalcemia after discontinuation of long-term denosumab treatment. Osteoporos Int 2016;27:2383-6. [Crossref] [PubMed]
- Lewiecki EM. Bisphosphonates for the treatment of osteoporosis: insights for clinicians. Ther Adv Chronic Dis 2010;1:115-28. [Crossref] [PubMed]
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756-65. [Crossref] [PubMed]
- Glorieux FH, Ward LM, Rauch F, et al. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res 2002;17:30-8. [Crossref] [PubMed]
- Parisi MS, Oliveri B, Mautalen CA. Effect of intravenous pamidronate on bone markers and local bone mineral density in fibrous dysplasia. Bone 2003;33:582-8. [Crossref] [PubMed]
- Palmisano B, Spica E, Remoli C, et al. RANKL Inhibition in Fibrous Dysplasia of Bone: A Preclinical Study in a Mouse Model of the Human Disease. J Bone Miner Res 2019;34:2171-82. [Crossref] [PubMed]
- Meier ME, van der Bruggen W, van de Sande MAJ, et al. Regression of fibrous dysplasia in response to denosumab therapy: A report of two cases. Bone Rep 2021;14:101058. [Crossref] [PubMed]
- Collins MT, de Castro LF, Boyce AM. Denosumab for Fibrous Dysplasia: Promising, but Questions Remain. J Clin Endocrinol Metab 2020;105:e4179-80. [Crossref] [PubMed]
- Martinez V, Sissons HA. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer 1988;61:2291-304. [Crossref] [PubMed]
- Vergel De Dios AM, Bond JR, Shives TC, et al. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer 1992;69:2921-31. [Crossref] [PubMed]
- Oliveira AM, Chou MM. USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol 2014;45:1-11. [Crossref] [PubMed]
- Oliveira AM, Perez-Atayde AR, Dal Cin P, et al. Aneurysmal bone cyst variant translocations upregulate USP6 transcription by promoter swapping with the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene 2005;24:3419-26. [Crossref] [PubMed]
- Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res 2015;101:S119-27. [Crossref] [PubMed]
- Muratori F, Mondanelli N, Rizzo AR, et al. Aneurysmal Bone Cyst: A Review of Management. Surg Technol Int 2019;35:325-35. [PubMed]
- Shiels WE 2nd, Mayerson JL. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report. Clin Orthop Relat Res 2013;471:2675-83. [Crossref] [PubMed]
- Park HY, Yang SK, Sheppard WL, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med 2016;9:435-44. [Crossref] [PubMed]
- Alhumaid I, Abu-Zaid A. Denosumab Therapy in the Management of Aneurysmal Bone Cysts: A Comprehensive Literature Review. Cureus 2019;11:e3989. [Crossref] [PubMed]
- Dunnion S, Paterson A, Johnston R. Dense sclerotic metaphyseal bands caused by denosumab therapy. Pediatr Radiol 2020;50:877-8. [Crossref] [PubMed]
- van der Heijden L, Dijkstra PDS, Blay JY, et al. Giant cell tumour of bone in the denosumab era. Eur J Cancer 2017;77:75-83. [Crossref] [PubMed]
- Knochentumoren Arbeitsgemeinschaft. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am 2008;90:1060-7. [Crossref] [PubMed]
- Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1-7. [Crossref] [PubMed]
- Chawla S, Blay JY, Rutkowski P, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol 2019;20:1719-29. [Crossref] [PubMed]
- Hoch B, Inwards C, Sundaram M, et al. Multicentric giant cell tumor of bone. Clinicopathologic analysis of thirty cases. J Bone Joint Surg Am 2006;88:1998-2008. [Crossref] [PubMed]
- Balke M, Schremper L, Gebert C, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol 2008;134:969-78. [Crossref] [PubMed]
- Tsukamoto S, Mavrogenis AF, Kido A, et al. Current Concepts in the Treatment of Giant Cell Tumors of Bone. Cancers (Basel) 2021;13:3647. [Crossref] [PubMed]
- Liede A, Bach BA, Stryker S, et al. Regional variation and challenges in estimating the incidence of giant cell tumor of bone. J Bone Joint Surg Am 2014;96:1999-2007. [Crossref] [PubMed]
- Kim TS, Usera GL, Ruggiero SL, et al. Improvement of Giant Cell Lesions of the Jaw Treated With High and Low Doses of Denosumab: A Case Series. JBMR Plus 2017;1:101-6. [Crossref] [PubMed]
- Upfill-Brown A, Bukata S, Bernthal NM, et al. Use of Denosumab in Children With Osteoclast Bone Dysplasias: Report of Three Cases. JBMR Plus 2019;3:e10210. [Crossref] [PubMed]
- Bredell M, Rordorf T, Kroiss S, et al. Denosumab as a Treatment Alternative for Central Giant Cell Granuloma: A Long-Term Retrospective Cohort Study. J Oral Maxillofac Surg 2018;76:775-84. [Crossref] [PubMed]
- Rhou YJJ, Wang CJ, Nguyen M, et al. Clinical and Radiologic Response of Central Giant Cell Granuloma to Denosumab: A 6-Year Prospective Observational Study. Calcif Tissue Int 2022;110:464-74. [Crossref] [PubMed]
- Choe M, Smith V, Okcu MF, et al. Treatment of central giant cell granuloma in children with denosumab. Pediatr Blood Cancer 2021;68:e28778. [Crossref] [PubMed]
- Mariz BALA, Migliorati CA, Alves FA, et al. Successful denosumab treatment for central giant cell granuloma in a 9-year-old child. Spec Care Dentist 2021;41:519-25. [Crossref] [PubMed]
- Grasemann C, Schündeln MM, Hövel M, et al. Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget's disease. J Clin Endocrinol Metab 2013;98:3121-6. [Crossref] [PubMed]
- Scheinberg MA, Golmia RP, Sallum AM, et al. Bone health in cerebral palsy and introduction of a novel therapy. Einstein (Sao Paulo) 2015;13:555-9. [Crossref] [PubMed]
- Ishida T, Yoshida S, Fujiki Y, et al. Effects of denosumab on rheumatic diseases and refractory glucocorticoid-induced osteoporosis: a prospective study. Arch Osteoporos 2021;16:39. [Crossref] [PubMed]
- Anastasilakis AD, Toulis KA, Polyzos SA, et al. Long-term treatment of osteoporosis: safety and efficacy appraisal of denosumab. Ther Clin Risk Manag 2012;8:295-306. [Crossref] [PubMed]
- Huang TH, Liu HC, Hou JY, et al. Efficacy and safety of denosumab therapy for low bone mineral density in childhood cancer survivors: A report of preliminary experience. Pediatr Blood Cancer 2019;66:e27927. [Crossref] [PubMed]
- Kumaki D, Nakamura Y, Sakai N, et al. Efficacy of Denosumab for Glucocorticoid-Induced Osteoporosis in an Adolescent Patient with Duchenne Muscular Dystrophy: A Case Report. JBJS Case Connect 2018;8:e22. [Crossref] [PubMed]
- Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999;397:315-23. [Crossref] [PubMed]
- Hoyer-Kuhn H, Semler O, Schoenau E. Effect of denosumab on the growing skeleton in osteogenesis imperfecta. J Clin Endocrinol Metab 2014;99:3954-5. [Crossref] [PubMed]
- Zeitlin L, Rauch F, Plotkin H, et al. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics 2003;111:1030-6. [Crossref] [PubMed]
- Burckhardt P, Faouzi M, Buclin T, et al. Fractures After Denosumab Discontinuation: A Retrospective Study of 797 Cases. J Bone Miner Res 2021;36:1717-28. [Crossref] [PubMed]
- Zheng S, Gaitonde P, Andrew MA, et al. Model-based assessment of dosing strategies in children for monoclonal antibodies exhibiting target-mediated drug disposition. CPT Pharmacometrics Syst Pharmacol 2014;3:e138. [Crossref] [PubMed]


