
Early metabolic derangements and unfavorable outcomes in pediatric traumatic brain injury: a retrospective multi-center cohort study
Highlight box
Key findings
• Early-onset, late-onset and persistent hyperglycemia are associated with unfavorable functional outcomes in pediatric traumatic brain injury (TBI).
What is known and what is new?
• Admission hyperglycemia is an important predictor of mortality in pediatric TBI. Limited studies have discussed the role of lactate and acidosis as prognostic markers in pediatric TBI.
• Hyperglycemia (early-onset, late-onset and persistent) in TBI were associated with unfavorable functional outcomes, while lactate levels and presence of acidosis were not.
What is the implication, and what should change now?
• Future trials should investigate the causal relationship between glycemic trends and functional outcomes in pediatric TBI.
• This will have implications for developing detailed protocols optimizing glycemic control in this cohort.
Introduction
Despite increased accessibility to healthcare resources and advancements in trauma resuscitation, pediatric traumatic brain injury (TBI) remains a significant health burden (1). In addition to optimization of hemodynamic parameters to reduce the chance of secondary brain damage, early identification and correction of biochemical alterations are vital (2-5).
Children with moderate and severe TBI can present with significant metabolic derangements characterized by hyperglycemia, hyperlactatemia and acidosis (4,6). The acute stress-induced response to injury constitutes a complex interplay of counteracting hormones, such as cortisol, glucagon, catecholamines and regulatory cytokines (7). This leads to impaired aerobic glycolysis and intracellular acidosis that in turn cause a secondary toxic-ischemic effect on the brain (7). The association between these metabolic alterations and outcomes of pediatric TBI have been investigated in several single-center studies (2,5,8,9).
These prior single-center studies have identified admission hyperglycemia as an important predictor of mortality in pediatric TBI (9-14). Early hyperglycemia has been traditionally described as the presence of any hyperglycemia in the first 48 h of injury (13,14), while other retrospective cohort studies have proposed a shorter window between 12 to 24 h post injury (5,15). The association between early, compared to late or persistent hyperglycemia and pediatric TBI outcomes has not been well-established (9-14). Additionally, mortality has been the primary outcome of interest in these studies whereas functional outcome has been less adequately described (9-14). Furthermore, while lactate and acidosis in adult multisystem trauma are associated with massive hemorrhage and prolonged ICU admission (16,17), there is a paucity of literature examining their roles as prognostic markers in pediatric TBI (2,4).
To address some of these gaps in current literature, we set out to investigate the association between glycemic trends throughout the first 72 h of pediatric intensive care unit (PICU) admission with unfavorable functional outcome defined by pediatric cerebral performance category (PCPC) categories of moderate disability, severe disability, vegetative state or coma (18,19), and death. We also sought to examine the association between hyperlactatemia and acidosis with unfavorable outcome in a large Asian, pediatric TBI cohort. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-443/rc).
Methods
Study design, setting and population
We performed a secondary analysis of the retrospective, multi-center Pediatric Acute and Critical Care Medicine Asian Network (PACCMAN) TBI data set, involving 10 pediatric intensive care units from January 2014 to October 2017 (20). This was a multi-national population that included 380 children <16 years old with a Glasgow Coma Scale (GCS) Score £13, and who presented within 24 h of head injury. We included patients with both isolated TBI and TBI in the presence of poly-trauma—defined as the presence of other extracranial injuries including intra-thoracic, intra-abdominal injuries and long bone fractures. Following the initial publication, we aimed to investigate early post traumatic seizures in children (5), as well as biochemical alterations in TBI. Among the 10 centers, 8 centers responded to our call and were able to provide further data on existing patients. Data were obtained using a standardized electronic REDCap data collection form.
Variables and definitions
Demographic details were collected, including age, gender, mechanism of injury and presenting GCS score on arrival to each institution. We reviewed presenting (initial) arterial glucose levels, serum lactate and pH levels as well as peak values recorded at 0–24, 24–48 and 48–72 h. Being an observational study, we did not standardize the frequency of blood-taking. Hyperglycemia was defined as a single glucose reading >11.1 mmol/L and hypoglycemia was defined as glucose <4.0 mmol/L (3,14). We defined early hyperglycemia as the presence of hyperglycemia within the first 24 h of admission to the PICU. As part of a sensitivity analysis, we repeated our analysis with the presence of hyperglycemia within the first 48 h of admission to the PICU. Late-onset hyperglycemia was defined as hyperglycemia that occurred beyond 48 h of admission to the PICU, and persistent hyperglycemia was defined as hyperglycemia that lasted throughout the first 72 h of admission. We also recorded the presence of insulin administration in the first 72 h. Hyperlactatemia was defined as lactate >2 mmol/L (2,4), and acidosis was defined as pH <7.35 (4).
Outcome measures
Our primary outcome of interest was functional outcome on discharge from the PICU, measured by PCPC, assigned by trained clinicians in the PICU of each institution. The PCPC is a qualitative assessment of function that consists of six categories: good, mild disability, moderate disability, severe disability, vegetative state or coma, and death (18,19). Unfavorable outcome was defined as PCPC categories of moderate disability, severe disability, vegetative state or coma, and death. We defined death as in-hospital mortality. Secondary outcomes included length of ventilation, length of stay (LOS) in PICU and LOS in hospital.
Statistical analysis
Data were collected and entered into a standardized Microsoft Excel database (Microsoft Corporation, New Mexico, the United States). Statistical analysis was performed using IBM SAS Statistics (SAS Institute, North Carolina, United States). Categorical data were summarized by frequencies and percentages, while continuous variables were expressed as median with interquartile range (IQR). We performed a univariate logistic regression analysis to determine if specific demographic factors, such as gender and age, alongside each laboratory marker—glucose, lactate and pH—predicted for unfavorable outcome. Variables subsequently included in the multivariable regression were determined based on univariate significance as well as their clinical relevance. Receiver Operating Characteristic (ROC) curves were drawn to compare the areas under the curve (AUC) for initial serum glucose, lactate and pH. The Youden index was used to identify thresholds for the aforementioned metabolic markers in determining unfavorable PCPC outcome, where the highest Youden Index were a summary measure of the ROC curve at which both the sensitivity and specificity of the biomarker is maximally optimized. We presented point estimates using unadjusted and adjusted odds ratios (aOR) together with the corresponding 95% confidence intervals (95% CI), and took statistical significance at P<0.05.
Ethics
Ethics approval was given for this study by SingHealth Centralised Institutional Review Board E, Singapore (No. 2018/2076) with waiver of informed consent due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Three hundred and thirteen patients were enrolled into the study, with a median age of 4.2 (IQR, 1.8–8.8) years. Eight patients (2.2%) were excluded due to incomplete outcome measurements of PCPC scoring on discharge.
Patient demographic
A total of 305 children were eligible for final analysis, of whom 207 (67.9%) were male. The most common mechanisms of injury were road traffic accidents (RTA) (133/305, 43.6%) and falls (133/305, 43.6%) (Table 1). Amongst the 305 children analyzed for the study, there were 2.2% (7/305) with incomplete glucose values, 18.6% (57/305) with missing lactate values and 1.9% (6/305) with missing pH values. There were 136 children (44.6%) with unfavorable PCPC outcome, of whom 102 children (75.0%) sustained severe TBI with a presenting GCS score of ≤8 (Table 1). A greater proportion of children with unfavorable outcome had sustained polytrauma compared to those who had favorable outcome (94/136, 69.1% vs. 80/169, 47.3%; P<0.001) (Table 1).
Table 1
| Clinical variable | Total (n=305) | Patients with favorablea PCPC outcome (n=169) | Patients with unfavorableb PCPC outcome (n=136) | P value+ |
|---|---|---|---|---|
| Age (years), median (IQR) | 4.2 (1.8–8.8) | 4.3 (1.8–8.6) | 3.6 (1.8–9.0) | 0.807 |
| Gender (males), n (%) | 207 (67.9) | 121 (71.6) | 86 (63.2) | 0.029 |
| Severe TBI with Glasgow Coma Scale ≤8, n (%) | 169 (55.4) | 67 (39.6) | 102 (75.0) | <0.001 |
| Mechanism of injury, n (%) | 0.054 | |||
| Road traffic accident | 133 (43.6) | 64 (37.9) | 69 (50.7) | |
| Fall | 133 (43.6) | 81 (47.9) | 52 (38.3) | |
| Non-accidental injury* | 22 (7.2) | 13 (7.7) | 9 (6.6) | |
| Others | 17 (5.6) | 11 (6.5) | 6 (4.4) | |
| Presence of polytrauma, n (%) | 174 (57.0) | 80 (47.3) | 94 (69.1) | <0.001 |
| First presenting glucose (mmol/L), median (IQR) | 8.4 (6.5–12.1) | 7.9 (6.3–9.6) | 9.5 (6.9–14.8) | <0.001 |
| Admission hyperglycemia >11.1 mmol/L, n (%) | 76 (24.9) | 19 (11.2) | 57 (41.9) | <0.001 |
| Early hyperglycemia >11.1mmol/L in first 24 h, n (%) | 108 (36.2) | 33 (19.5) | 75 (55.1) | <0.001 |
| Presence of persistent hyperglycemia >11.1 mmol/L throughout first 72 h, n (%) | 5 (1.6) | 0 (0.0) | 5 (3.7) | 0.179 |
| Presence of late-onset hyperglycemia >11.1 mmol/L beyond 48 h, n (%) | 13 (4.3) | 1 (0.6) | 12 (8.8) | 0.239 |
| Admission lactate (mmol/L), median (IQR) | 2.2 (1.3–4.0) | 1.9 (1.2–3.4) | 2.7 (1.5–5.2) | <0.001 |
| Presence of early hyperlactatemia >2 mmol/L in the first 24 h, n (%) | 130 (42.3) | 56 (32.5) | 74 (54.4) | 0.013 |
| Admission pH, median (IQR) | 7.30 (7.27–7.41) | 7.35 (7.31–7.40) | 7.31 (7.23–7.43) | 0.003 |
| Presence of early acidosis pH <7.35 in first 24 h, n (%) | 163 (53.4) | 78 (46.1) | 85 (62.5) | 0.003 |
a, favorable PCPC outcome defined as PCPC categories of good function and mild disability; b, unfavorable PCPC outcome defined as PCPC categories of moderate disability, severe disability, vegetative state or coma or brain death; +, statistical significance taken at P<0.05; *, non-accidental injury is defined as injury that is purposefully inflicted onto the child, including any violent physical act. PCPC, pediatric cerebral performance category; TBI, traumatic brain injury; IQR, interquartile range.
Association of hyperglycemia with clinical outcome
On admission to PICU, there were 76 patients (24.9%) with hyperglycemia, 217 (74.0%) with normoglycemia and 5 (1.7%) with hypoglycemia. There were seven patients without glucose values recorded. The median glucose level on admission was 8.4 (IQR, 6.5–12.1) mmol/L (P<0.001). There were 108 patients (36.2%) with early hyperglycemia within the first 24 h of PICU admission (Table 1). Amongst the 108 patients with early hyperglycemia, 15 (13.8%) received insulin therapy within the first 24 h, of whom 7 patients continued to remain hyperglycemic at 24–48 h of admission.
Early hyperglycemia and clinical outcome
The presence of early hyperglycemia in the first 24 h of PICU admission was associated with unfavorable PCPC outcome (75/108, 69.4% vs. 59/187, 31.5%, P<0.001) compared to the group with normoglycemia (Table 2). The cohort with early hyperglycemia had an increased length of ICU stay compared to those with normoglycemia [8.5 (IQR, 4–14) vs. 6 (IQR, 3–10) days; P=0.004) (Table 2). Early hyperglycemia in the first 24 h, however, was not associated with a longer duration of ventilation [5 (IQR, 2–9) vs. 4 (IQR, 2–8) days; P=0.186] or duration of hospitalization [23 (IQR, 9–35) vs. 14 (IQR, 8–29) days; P=0.233] compared to the normoglycemia group (Table 2). In our sensitivity analysis, we found a consistent association between early hyperglycemia (when defined within the first 48 h) and unfavorable outcome (Table S1).
Table 2
| Clinical outcome | Normoglycemia within first 24 h (glucose ≤11.1 mmol/L) (n=187) | Early hyperglycemia in first 24 h (glucose >11.1 mmol/L) (n=108) | P value |
|---|---|---|---|
| LOS of hospital (day), median (IQR) | 14 (8, 29) | 23 (9, 35) | 0.233 |
| LOS of PICU (day), median (IQR) | 6 (3, 10) | 8.5 (4, 14) | 0.004 |
| Duration of mechanical ventilation, median (IQR) | 4 (2, 8) | 5 (2, 9) | 0.186 |
| Discharge PCPC rating, n (%) | <0.001 | ||
| Good | 81 (43.3) | 17 (15.7) | |
| Mild disability | 47 (25.1) | 16 (14.8) | |
| Moderate disability* | 22 (11.8) | 15 (13.8) | |
| Severe disability* | 18 (9.6) | 11 (10.1) | |
| Vegetative state or coma* | 15 (8.0) | 29 (26.8) | |
| Death* | 4 (2.1) | 20 (18.5) |
*, unfavorable functional outcome is defined as PCPC scale ratings of moderate disability, severe disability and vegetative state or coma, death. PCPC scores assigned as: 1, good; 2, mild disability; 3, moderate disability; 4, severe disability; 5, vegetative state or coma; 6, death. LOS, length of stay; IQR, interquartile range; PICU, pediatric intensive care unit; PCPC, pediatric cerebral performance category.
After adjusting for gender, presenting GCS £8 and presence of polytrauma, early hyperglycemia was associated with unfavorable functional outcome (aOR =3.68, 95% CI: 2.12–6.39, P<0.001) (Table 3). At the maximum Youden Index, we observed an initial glycemic threshold of >11.0 mmol/L to be associated with unfavorable outcome with a sensitivity of 56.2% and specificity of 79.8% (Figure S1A).
Table 3
| Variable | Adjusted odds ratio (95% confidence interval) | P value |
|---|---|---|
| Male gender | 1.79 (1.02–3.11) | 0.039 |
| Glasgow Coma Score ≤8 | 2.84 (1.65–4,88) | 0.008 |
| Presence of polytrauma | 2.06 (1.20–3.51) | <0.001 |
| Early hyperglycemia >11.1 mmol/L | 3.68 (2.12–6.39) | <0.001 |
*, unfavorable functional outcome defined as PCPC scale ratings of moderate disability, severe disability and vegetative state or coma, death. PCPC, pediatric cerebral performance category.
Late-onset hyperglycemia and clinical outcome
Amongst 13 patients who developed late-onset hyperglycemia at 48–72 h of admission, 12 (92.3%) had unfavorable outcome. We found that the presence of late-onset hyperglycemia was associated with unfavorable outcome (aOR =13.30, 95% CI: 1.64–107.8, P=0.015) (Table 4). We were unable to perform multivariable analyses for the subgroup with persistent hyperglycemia as all progressed to have unfavorable outcomes.
Table 4
| Variable | Adjusted odds ratio (95% confidence interval) | P value |
|---|---|---|
| Male gender | 1.53 (0.83–2.81) | 0.170 |
| Glasgow Coma Score ≤8 | 2.07 (1.15–3.74) | 0.015 |
| Presence of polytrauma | 1.84 (1.01–3.36) | 0.046 |
| Late-onset hyperglycemia >11.1 mmol/L | 13.30 (1.64–107.8) | 0.015 |
*, unfavorable functional outcome defined as PCPC scale ratings of moderate disability, severe disability and vegetative state or coma, death. PCPC, pediatric cerebral performance category.
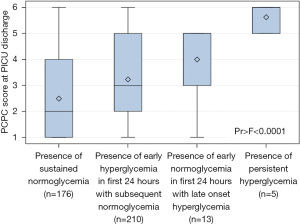
We found that children who maintained normoglycemia from presentation to 72 h of admission had favorable functional outcome with a median PCPC score of 2 on discharge (IQR, 1–4; P<0.001) (Figure 1). Normoglycemic children who later developed hyperglycemia beyond 48 hours of admission progressed to have worse outcome than children with early hyperglycemia that normalized after 24 h [median PCPC score 5 (IQR, 3–5) vs. PCPC score 3 (IQR, 2–5); P<0.001] (Figure 1). Finally, children with persistent hyperglycemia throughout the first 72 h of admission progressed to have poorest outcomes with a median PCPC score of 6 (IQR, 5–6; P<0.001), corresponding to death (Figure 1).
Association of lactatemia and acidosis with clinical outcome
Next, we analyzed the association between other deranged metabolic markers with clinical outcome. Patients with unfavorable outcome presented with a higher median admission lactate than the group with favorable outcome [2.7 (IQR, 1.5–5.2) vs. 1.9 (IQR, 1.2–3.4) mmol/L; P<0.001] (Table 1). Table 5 shows clinical outcomes between children with high lactate >2.0 mmol/L in the first 24 h compared to those without. There was a greater proportion with moderate disability, severe disability, vegetative state or coma, and death cumulatively in those with high lactate compared to those without (74/130, 56.9% compared to 53/121, 43.8%; P<0.001) (Table 5). This was especially observed at lactate levels >3.8 mmol/L (AUC =0.65, OR =1.26, 95% CI: 1.13–1.41, P<0.001) (Figure S1B). The presence of acidosis was also similarly associated with unfavorable PCPC outcome (P=0.004) (Table 6).
Table 5
| Clinical outcome | Normal lactate levels in the first 24 h (lactate ≤2.0 mmol/L) (n=121) | Early hyperlactatemia in the first 24 h (lactate >2.0 mmol/L) (n=130) | P value |
|---|---|---|---|
| LOS of hospital (day), median [IQR] | 14.5 [8.5–30] | 23 [10–38.5] | 0.171 |
| LOS of PICU (day), median [IQR] | 7 [4–12] | 8 [4–1] | 0.774 |
| Duration of mechanical ventilation, median [IQR] | 5 [2–8] | 5 [2–9] | 0.806 |
| Discharge PCPC rating, n (%) | <0.001 | ||
| Good | 34 (28.6) | 31 (24.0) | |
| Mild disability | 32 (26.9) | 24 (18.6) | |
| Moderate disability* | 21 (17.6) | 12 (9.3) | |
| Severe disability* | 16 (13.5) | 12 (9.3) | |
| Vegetative state or coma* | 12 (10.1) | 32 (24.8) | |
| Death* | 4 (3.4) | 18 (14.0) |
*, unfavorable functional outcome is defined as PCPC scale ratings of moderate disability, severe disability and vegetative state or coma, death. PCPC scores assigned as: 1, good; 2, mild disability; 3, moderate disability; 4, severe disability; 5, vegetative state or coma; 6, death. LOS, length of stay; IQR, interquartile range; PICU, pediatric intensive care unit; PCPC, pediatric cerebral performance category.
Table 6
| Clinical outcome | Normal pH levels (pH ≥7.35) within the first 24 h (n=135) | Early acidosis (pH <7.35) within the first 24 h (n=163) | P value |
|---|---|---|---|
| LOS of hospital (day), median [IQR] | 15 [8–26] | 17 [8.5–35] | 0.323 |
| LOS of PICU (day), median [IQR] | 6 [4–11] | 7 [4–13] | 0.278 |
| Duration of mechanical ventilation, median [IQR] | 4 [2–7] | 5 [2–9] | 0.439 |
| Discharge PCPC rating, n (%) | 0.004 | ||
| Good | 48 (35.6) | 51 (31.3) | |
| Mild disability | 37 (27.4) | 27 (16.6) | |
| Moderate disability* | 20 (14.8) | 17 (10.4) | |
| Severe disability* | 9 (6.7) | 21 (12.9) | |
| Vegetative state or coma* | 17 (12.6) | 26 (16.0) | |
| Death* | 4 (3.0) | 21 (12.9) |
*, unfavorable functional outcome is defined as PCPC scale ratings of moderate disability, severe disability and vegetative state or coma, death. PCPC scores assigned as: 1, good; 2, mild disability; 3, moderate disability; 4, severe disability; 5, vegetative state or coma; 6, death. LOS, length of stay; IQR, interquartile range; PICU, pediatric intensive care unit; PCPC, pediatric cerebral performance category.
Finally, we compared these three metabolic markers and their association with unfavorable outcome. In our ROC model, initial glucose values in the first 24 h had the highest AUC in comparison to initial lactate and pH (0.70 vs. 0.65 and 0.59, respectively) (Figure S1A-S1C). Initial hyperglycemia was also the metabolic marker with highest sensitivity (56.2%) for unfavorable outcome in comparison to initial hyperlactatemia (48.8%) and initial acidosis (45.1%). In our multivariable analysis, after adjusting for gender, presence of polytrauma and concomitant presence of admission hyperglycemia, hyperlactatemia and acidosis were not associated with unfavorable outcomes (Tables S2,S3).
Discussion
In this retrospective, multi-center study, we analyzed 305 pediatric patients with moderate to severe TBI. We described a young cohort with a median age of 4.2 years, whose predominant mechanism of injury was RTA, consistent with previous studies (1,14,21,22). Amongst our patients, 44.6% had unfavorable outcome. We found early hyperglycemia to be independently associated with unfavorable functional outcome, while lactate levels and presence of acidosis were not.
Numerous studies have investigated serum glucose as a prognostic marker in TBI (5,10,11,14,15). We followed prior thresholds for hyperglycemia, conventionally defined as glucose ≥11.1 mmol/L (5,12,23). More recent studies however, have since sought to determine if this glycemic threshold should be lowered and if tight glycemic control will lead to better outcomes (14,15). Adult TBI studies reported that tight glycemic control did not improve clinical outcomes (24,25). However, interventional studies instituting strict glycemic control in pediatric TBI patients are lacking, and we postulate that this is because clinicians are concerned about the inadvertent risks of hypoglycemia if glucose control is too stringent (14). We found that the conventional glucose cut off at 11.0 mmol/L—corresponding to the highest Youden index—was indeed associated with unfavorable PCPC outcome in our population. However, because of the retrospective nature of our study, glucose levels were not continuously measured nor measured at protocolized timing, and our results should be interpreted with caution.
Past studies of hyperglycemia in both adult and pediatric TBI had used differing definitions for early hyperglycemia, with larger studies conventionally using a 48-h window (13,14,26). While arguably transient and possibly a reflection of the pathophysiological stress response immediately post-insult (5), several studies have demonstrated that early hyperglycemia in the first 24 h was associated with adverse outcome in both adult and pediatric TBI populations (12,23,24). A large retrospective cohort study (n=271) at a tertiary pediatric trauma center in 2014 demonstrated that severe hyperglycemia during the initial 12 hours was independently associated with both unfavorable outcome and mortality in pediatric TBI (15). We chose to define the early hyperglycemic period within the first 24 h based on the data extrapolated from the above. Our findings reiterate extant knowledge—patients with early hyperglycemia in the first 24 h had longer duration of PICU stay and increased mortality. Amongst the survivors, our patients also progressed to have unfavorable functional outcomes. Of note, our findings were in conflict with a retrospective study performed at a single pediatric tertiary center by Smith et al. in 2012, which found no association between early hyperglycemia with outcome (14). We postulate that this could be due to variation in fluids use, treatment thresholds for administration of insulin, and concurrent TBI management strategies that impact outcomes.
The pathophysiology of the glycemic trend that follows in the next 48–72 h is a lesser understood phenomenon constituted by a complex interplay of metabolic processes involving secondary brain tissue inflammation and injury (27-29). It is proposed that altered glucose metabolism and mitochondrial dysfunction occurs in later phases of brain injury, leading to hyperglycemia presenting beyond the first 24 h of injury (28). Smith et al. had reported in their retrospective, single-centre study that delayed hyperglycemia after 48 h post injury was associated with adverse outcomes amongst children with severe TBI (14). Similarly, we described late-onset hyperglycemia as a strong independent predictor of unfavorable outcome in addition to early hyperglycemia alone. Notably, all five patients with persistent hyperglycemia died. We believe that our observations merit further large-scale multi-center studies to investigate outcomes in pediatric TBI patients with late-onset, and sustained hyperglycemia.
There are limited studies examining the association between lactate, acidosis, and outcomes (2). While these biomarkers reflect the extent of tissue hypoperfusion and hypoxia in trauma, their role particularly in isolated brain injury is not clearly established (2). In 2019, a single-center retrospective study (n=213) reported an association between serum lactate on admission with decreased ventilation-free, PICU-free and hospital-free days in children with moderate and severe TBI (2). We were, however, unable to demonstrate this association in our study. Serum lactate and acidosis were associated with unfavorable PCPC outcome in our univariate analysis, but not for duration of ventilation, PICU stay and duration of hospitalization. After accounting for the concurrent presence of hyperglycemia and adjusting for gender and presence of polytrauma, we did not find any association between both lactate and acidosis with unfavorable outcome. We recognize that not all patients had documented lactate trends and methods used to collect lactate samples may have varied across sites. Interventions were also likely to have been taken to elucidate the cause and/or correct these abnormalities.
The primary limitation of our study is its retrospective design, although initial data was actively captured in each institution’s trauma surveillance database. We recognize that the frequency of monitoring of these metabolic markers, and thresholds for intervention were likely to differ across sites and some confounders may not be known. In our current study, the percentage of missing data was 2.2%; these were removed from our regression model analysis. Additionally, over the period in which we utilized these data, there were evolving clinical practices involving ventilator management, temperature control and intracranial pressure control strategies that may have varied internationally. We were unable to obtain details on the number of patients who had cardiac arrest as a complication of their TBI, steroid treatment, use of etomidate at time of intubation, the maximum glucose infusion rate of intravenous fluids used, and information on nutrition initiation including the use of total parental nutrition, all of which would have had implications on glycaemia—particularly in the late-onset hyperglycemic cohort. Given the small number of children with late-onset and/or persistent hyperglycemia in our study, we recognize that our results must be interpreted with caution. Finally, while we used PCPC scoring on discharge as the end-point assessment for our patients’ functional outcome, we recognize that PCPC scores may change with neuro-rehabilitation, post discharge.
Conclusions
In this multi-center pediatric TBI cohort, early hyperglycemia in the first 24 h, late-onset and persistent hyperglycemia were incrementally associated with unfavorable functional outcomes. Future trials should investigate the causal relationship between glycemic trends and functional outcomes in this cohort, and if glycemic control alters this relationship.
Acknowledgments
Funding: This study was funded by the SingHealth Foundation Research Grant (No. SHF/FG670P/2017).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-443/rc
Data Sharing Statement: https://tp.amegroups.com/article/view/10.21037/tp-22-443/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-443/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-443/coif). JHL serves as an unpaid Deputy Editor-in-Chief of Translational Pediatrics from July 2022 to June 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was given for this study by SingHealth Centralised Institutional Review Board E, Singapore (No. 2018/2076) with waiver of informed consent due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bowman SM, Bird TM, Aitken ME, et al. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatrics 2008;122:988-93. [Crossref] [PubMed]
- Fu YQ, Bai K, Liu CJ. The impact of admission serum lactate on children with moderate to severe traumatic brain injury. PLoS One 2019;14:e0222591. [Crossref] [PubMed]
- Chiaretti A, Piastra M, Pulitanò S, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst 2002;18:129-36. [Crossref] [PubMed]
- Ng ZM, Hong WJ, Chong SL, et al. Correlation of arterial blood gas markers and lactate levels with outcomes in pediatric traumatic brain injury. Proc Singapore Healthc 2017;26:201010581770420. [Crossref]
- Chong SL, Harjanto S, Testoni D, et al. Early Hyperglycemia in Pediatric Traumatic Brain Injury Predicts for Mortality, Prolonged Duration of Mechanical Ventilation, and Intensive Care Stay. Int J Endocrinol 2015;2015:719476. [Crossref] [PubMed]
- McKenna MC, Scafidi S, Robertson CL. Metabolic Alterations in Developing Brain After Injury: Knowns and Unknowns. Neurochem Res 2015;40:2527-43. [Crossref] [PubMed]
- Makoroff KL, Cecil KM, Care M, et al. Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr Radiol 2005;35:668-76. [Crossref] [PubMed]
- Han J, Lee YI, Ryu JA. Prognostic Value of Early Hyperglycemia in Neurocritically Ill Patients. J Neurointensive Care 2020;3:6-11. [Crossref]
- Aşılıoğlu N, Turna F, Paksu MS. Admission hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. J Pediatr (Rio J) 2011;87:325-8. [Crossref] [PubMed]
- Chen S, Liu Z. Effect of hyperglycemia on all-cause mortality from pediatric brain injury: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e23307. [Crossref] [PubMed]
- Bellomy ML, Freundlich RE. Hyperglycemia and Elevated Lactate in Trauma: Where Do We Go From Here? Anesth Analg 2018;126:748-9. [Crossref] [PubMed]
- Cochran A, Scaife ER, Hansen KW, et al. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma 2003;55:1035-8. [Crossref] [PubMed]
- Melo JR, Di Rocco F, Blanot S, et al. Acute hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. Acta Neurochir (Wien) 2010;152:1559-65. [Crossref] [PubMed]
- Smith RL, Lin JC, Adelson PD, et al. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med 2012;13:85-91. [Crossref] [PubMed]
- Elkon B, Cambrin JR, Hirshberg E, et al. Hyperglycemia: an independent risk factor for poor outcome in children with traumatic brain injury*. Pediatr Crit Care Med 2014;15:623-31. [Crossref] [PubMed]
- Raux M, Le Manach Y, Gauss T, et al. Comparison of the Prognostic Significance of Initial Blood Lactate and Base Deficit in Trauma Patients. Anesthesiology 2017;126:522-33. [Crossref] [PubMed]
- Régnier MA, Raux M, Le Manach Y, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology 2012;117:1276-88. [Crossref] [PubMed]
- Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616-20. [Crossref] [PubMed]
- Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Crit Care Med 2000;28:1173-9. [Crossref] [PubMed]
- Chong SL, Dang H, Ming M, et al. Traumatic Brain Injury Outcomes in 10 Asian Pediatric ICUs: A Pediatric Acute and Critical Care Medicine Asian Network Retrospective Study. Pediatr Crit Care Med 2021;22:401-11. [Crossref] [PubMed]
- Tsai YW, Wu SC, Huang CY, et al. Impact of stress-induced hyperglycemia on the outcome of children with trauma: A cross-sectional analysis based on propensity score-matched population. Sci Rep 2019;9:16311. [Crossref] [PubMed]
- Kan CH, Saffari M, Khoo TH. Prognostic factors of severe traumatic brain injury outcome in children aged 2-16 years at a major neurosurgical referral centre. Malays J Med Sci 2009;16:25-33. [PubMed]
- Seyed Saadat SM, Bidabadi E, Seyed Saadat SN, et al. Association of persistent hyperglycemia with outcome of severe traumatic brain injury in pediatric population. Childs Nerv Syst 2012;28:1773-7. [Crossref] [PubMed]
- Vespa P, Boonyaputthikul R, McArthur DL, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med 2006;34:850-6. [Crossref] [PubMed]
- Hermanides J, Plummer MP, Finnis M, et al. Glycaemic control targets after traumatic brain injury: a systematic review and meta-analysis. Crit Care 2018;22:11. [Crossref] [PubMed]
- Liu-DeRyke X, Collingridge DS, Orme J, et al. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care 2009;11:151-7. [Crossref] [PubMed]
- Jalloh I, Carpenter KL, Helmy A, et al. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological findings. Metab Brain Dis 2015;30:615-32. [Crossref] [PubMed]
- Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care 2016;4:29. [Crossref] [PubMed]
- Robertson CL, Saraswati M, Scafidi S, et al. Cerebral glucose metabolism in an immature rat model of pediatric traumatic brain injury. J Neurotrauma 2013;30:2066-72. [Crossref] [PubMed]


