
Repair of proximal hypospadias with single-stage (Duckett’s method) or Bracka two-stage: a retrospective comparative cohort study
Highlight box
Key findings
• The Bracka two-stage repair is a safer and more reliable approach for proximal hypospadias in children. The Bracka two-stage repair should be used for perineal hypospadias. The larger the urethral defect after chordee correction, the greater the possibility of a postoperative urethral fistula.
What is known and what is new?
• Duckett’s method is a classic non-staging operation, and two-stage Bracka repair is an attractive alternative procedure.
• The efficacy of the Bracka two-stage and Duckett methods in the repair of proximal hypospadias was analyzed in addition to the potential risk factors for complications.
What is the implication, and what should change now?
• The two-stage Bracka repair can be applied in almost all types of proximal hypospadias with good cosmetic results, especially in cases of perineal hypospadias. The Bracka two-stage repair has low complication rates, and implementation of the Bracka procedure is straightforward if the surgeon has basic hypospadias repair skills.
Introduction
Hypospadias is a common congenital defect of the male external genitalia that occurs in approximately 1 in 250 live male newborns. Proximal hypospadias (penoscrotal, scrotal, and perineal types) account for approximately 20% of all cases (1). The management of hypospadias has greatly improved over the past two decades since the introduction of tubular incision plate urethroplasty. However, obtaining a favorable cosmetic outcome and functional straight penis is a major surgical challenge for such patients, and the ideal repair of proximal hypospadias remains the Holy Grail for hypospadias specialists. The surgical plan for proximal hypospadias can be divided into single and staged operations. Duckett repair is a classic single-stage procedure (2). Single-stage procedures are often associated with high rates of complications and reoperations, which defeats the initial purpose of a single-stage procedure. Reportedly, complications occur in 20–50% of patients with proximal hypospadias undergoing a contemporary series of single-stage repairs (3). Therefore, many pediatric surgeons are selecting staged procedures (4,5). Recently, staged repairing with inner preputial layer graft has regained popularity in the repair of proximal hypospadias (6,7).
Proximal hypospadias combined with severe chordee is a major challenge for pediatric surgeons and urologists. Staged surgical repair of proximal forms of hypospadias according to Bracka’s method has been proven to achieve successful both functional and cosmetic results. It is a versatile technique that is relatively easy to learn and applicable in difficult cases of salvage hypospadias. Alternatively, the Duckett procedure should be performed in one step because it is necessary to repair the chordee by transecting the plate. Although the Bracka two-stage and Duckett repair are both used by many hypospadias specialists, however, less information is available about the gap of therapeutic efficacy between the two surgical schemes. The present study aimed to compare the efficacy of the Bracka two-stage and Duckett methods in the repair of proximal hypospadias and to preliminarily analyze the potential risk factors for complications. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-75/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Tianjin Children’s Hospital (No. 2022-LXKY-001). Individual consent for this retrospective analysis was waived.
Study design and patients
We retrospectively reviewed the records of patients treated for hypospadias between January 2015 and January 2019 in Tianjin Children’s Hospital. Only patients with proximal hypospadias repair were included in the present analysis. In total, 745 patients underwent hypospadias repair during this period. We excluded those with incomplete medical records (n=31), distal and midshaft forms of hypospadias (n=565), and repairs using techniques other than Bracka and Duckett repair (n=53). Ultimately, 94 patients were included and assigned to two groups based on the technique used: 46 patients were treated with Bracka and 48 with Duckett. The choice of procedure is determined based on the preference of the parents of the patient after informing them of the advantages and disadvantages of the different procedures. We collected information regarding age at surgery, urethral meatus location, penis length, urethral defect before and after complete correction of chordee, ventral curvature (VC) before and after degloving, and follow-up time. The primary outcome of interest was the rate of postoperative complications.
Surgical technique
Common procedures
After anesthesia and disinfection, the penile head was pulled with a 5-0 Prolene suture. The length of the initial penis and distance of the urethral defect were measured. The penile curvature was measured after inducing an artificial erection. Subsequently, a circumferential incision was made proximal to the corona, and the coronal sulcus and penile skin was degloved to the root. Subsequently, the scar fiber around the corpus spongiosum was cut to release the chordee.. The curvature of the penis was then measured, and the urethral plate was transected at the point of severe chordee, and the urethral plate and the fibrous tissues were dissected “finely” from the proximal to distal end of the shaft until the penis was completely straightened. In case of incomplete correction, dorsal tunica albuginea plication was applied. After correction, the length of the penis and distance of the urethral defect were measured again.
Bracka two-stage repair
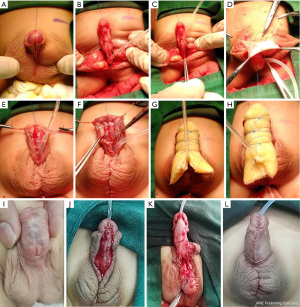
As shown in Figure 1, a circumferential incision was made along and 0.5 cm below the corona, reaching the depth of the Buck’s fascia (Figure 1A). The meatus was dropped back to the penile-scrotal junction, scrotum, or perineum (Figure 1B). If possible, we spatulated the new urethral meatus obliquely after creating a Duplay urethra with a retained urethral plate (Figure 1C). After harvesting the graft, the remaining subcutaneous tissue was removed, creating a thin translucent skin strip (Figure 1D). The dorsal foreskin was transferred to both sides of the penis (Figure 1E). Deformity of the bifid scrotum was corrected (Figure 1F). Petroleum gauze was rolled into a tube that covered an 8-Fr silicone catheter, inserted through the neourethra in advance, and then fixed properly to the ventral side of the penis (Figure 1G). A 1-cm incision was made across the urethral catheter on the proximal end of the petroleum gauze. Petroleum gauze was used as a compressive dressing to cover the graft and anastomosis snugly (Figure 1H). The graft became a stable, vascularized new urethral plate (approximately 1.5 cm wide) (Figure 1I). The neourethral plate was tubularized using a continuous 6-0 polyglactin suture (Figure 1J). After the neourethra was formed, a protective dartos fascia flap or tunica vaginalis flap was placed over the entire suture line as a waterproof layer (Figure 1K). Appearance of the penis after 2-staged operation (Figure 1L).
Duckett procedure
The operative procedures of Duckett were the same as those of the first stage of Bracka two-stage repair, and a protective dartos fascia flap or tunica vaginalis flap was placed over the entire suture line.
Prophylactic antibiotics were administered intravenously for 1 day in all patients. Postoperatively, a urethral catheter was used for 14 days, and penile gauze was used for 1 week. A bladder fistula was not established in either the one-stage nor the two-stage procedure. After removing the urinary tube, a sitz bath with warm saltwater was used as much as possible to promote wound healing and reduce swelling.
Follow-up and statistical analysis
Patients were advised to visit our urology clinic for outpatient follow-up examinations at 1 week, 1 month, 3 months, 6 months, 12 months, and once a year after the operation. Complications, including meatal stenosis, fistula formation, diverticula formation, stricture, and partial glans dehiscence, were found in both groups. If obstruction-related symptoms such as dysuria, straining, or repeated urinary tract infection were observed, immediate hospitalization was required.
Dichotomous variables were analyzed using the Chi-square test and continuous variables were analyzed using the t-test. Penis length, curvature, and urethral defect length were analyzed using one-way analysis of variance (ANOVA). All analyses were conducted using SPSS version 22.0 (IBM Corp., Armonk, NY, USA), with statistical significance set at P<0.05.
Results
In the Bracka group, the position of the urethral meatus was proximal penile type in 14 patients, penoscrotal type in 26, and perineal type in 6. In the Duckett group, the position was proximal penile type in 16 patients, penoscrotal type in 27, and perineal type in 5. The average age of the patients at surgery was 21.78 months (10–56 months) in the Bracka group and 22.23 months (9–76 months) in the Duckett group, and the mean follow-up durations were 30 months (24–64 months) and 38.8 months (35–70 months), respectively. Patient characteristics are presented in Table 1. There was no significant difference between the two groups in terms of age and type of hypospadias.
Table 1
| Variables | Bracka | Duckett | P |
|---|---|---|---|
| Age (months)# | 22.37±11.23 | 21.73±12.25 | 0.854 |
| Types of hypospadias, n (%) | 0.905 | ||
| Proximal penile | 14 (30.4) | 16 (33.3) | |
| Penoscrotal | 26 (56.5) | 27 (56.3) | |
| Perineal | 6 (13.1) | 5 (10.4) | |
#, results are presented as the mean ± standard deviation.
Patients in the Bracka group achieved complete penile straightening after the first stage. The new urethral plate was 1.5 cm wide, smooth, and adequately healed, with no scar healing or contracture. In perineal and scrotal hypospadias, after the formation of part of the proximal urethra, the new urethral meatus was located in the penoscrotal region. Meatal stenosis after the first stage was noted in 1 case (2.2%) in the Bracka group and was restored with ureteral dilatation. All patients in the Bracka group received second-stage repair, the appearance of the penis was satisfactory, and the urethral orifice was slit-like. However, fistulas were noted in 4 cases (8.7%) and urethral strictures in 2 cases (4.3%). In the Duckett group, fistulas were noted in 8 cases (16.7%), strictures in 4 cases (8.3%), partial glans dehiscence in 4 cases (8.3%), and diverticulum in 1 case (2.1%). Therefore, 6 cases (13.1%) in the Bracka group and 17 cases (35.4%) in the Duckett group required reoperation (Table 2). The incidence of postoperative complications in the Duckett group was significantly higher than in the Bracka group (P=0.016). Furthermore, compared with the Duckett group, children with perineal hypospadias who were treated with the Bracka operation had fewer postoperative complications (100% vs. 13%, P=0.015).
Table 2
| Groups | Fistula | Stricture | Dehiscence | Diverticulum | Reoperation |
|---|---|---|---|---|---|
| Bracka | 4 (8.7%) | 2 (4.3%) | 0 (0%) | 0 (0%) | 6 (13.1%) |
| Duckett | 8 (16.7%) | 4 (8.3%) | 4 (8.3%) | 1 (2.1%) | 17 (35.4%) |
| P | 0.356 | 0.678 | 0.117 | 0.969 | 0.016 |
In the Bracka and Duckett groups, the initial penis lengths were 3.59±0.49 versus 3.71±0.45 cm, and after complete chordee correction they were 4.50±0.45 versus 4.58±0.41 cm, respectively. The initial urethral defect in the two groups was 2.23±0.35 versus 2.27±0.36 cm, and it was 3.63±0.40 versus 3.51±0.42 cm after complete chordee correction. For penile curvature, the initial curvature was 43.69°±7.93° versus 41.81°±7.56°, and it was 30.17°±5.88° versus 29.65°±6.89° after degloving. There were no significant differences in the initial penis length, initial urethral defect, urethral defect after chordee correction, initial penile curvature, or penile curvature after degloving (P>0.05).
Analysis of the risk factors for postoperative complications revealed that surgery age, initial urethral defect, and initial curvature were not correlated with complications. There was a significant correlation between urethral defects after chordee correction and urethral percutaneous fistulas (P=0.019). Additionally, there was no significant correlation between the degree of penile curvature after degloving and urethral stenosis (P=0.058).
Discussion
Hypospadias is a common urogenital anomaly. The tubularized incised plate (TIP) procedure is considered an excellent choice for most cases of distal hypospadias with a light VC (8). It has a low incidence of stenosis resulting from preserved urethral plate and semicircular anastomosis without end-to-end anastomosis. The smooth urethral plate tissue is tightly adhered to the corpus cavernosum; therefore, the new urethra will not prolapse, thereby reducing the occurrence of a diverticulum. However, the urethral plate cannot be preserved during proximal hypospadias repair. After transecting the urethral plate to correct the VC, the urethral meatus retracts to the penile-scrotal junction, scrotum, or perineum. In the 1980s, it was common to repair proximal hypospadias with single-stage urethroplasty, but this procedure has a long learning curve and many complications (9,10). The main complications are urinary fistulas, urethral strictures, and diverticula.
In 1998, Snodgrass (11) first reported the TIP technique for proximal hypospadias for a selection of patients. Due to concerns over complications, resurgery, and cosmetic effects of single-stage repairs, Retik (12) reintroduced a two-stage repair for proximal hypospadias. A global survey through an anonymous online questionnaire showed that 43.3–76.6% of respondents preferred two-stage procedures for the repair of proximal hypospadias (13). Although two procedures were required, each operation was easy to master, the outcomes were satisfactory, and the occurrence of various complications was reduced.
The two-stage repair perfectly solved the problem of how to change the pathological features of proximal hypospadias. The first-stage operation straightened the penis and provided a healthier urethral plate for second-stage surgery. The first-stage operation could also reconstruct part of the proximal urethra and correct the bifid scrotum. No fistula occurred after the first-stage operation in the Bracka group, which may have resulted from extensive coverage of the perineum and scrotum by the new urethra with good blood supply. The urethral plate, closely attached to the corpus cavernosum, was perfectly reconstructed using a free foreskin inner graft with Bracka repair. A smooth and flat urethral plate is a prerequisite for urethroplasty success. This helps to increase the success rate and satisfaction with surgery. The second-stage operation is equivalent to the completion of the distal hypospadias operation with the TIP or Duplay techniques, and thus it can achieve the same success rate and satisfactory appearance as distal hypospadias.
Currently, there are three commonly used two-stage techniques available. The most traditional method is the Byars technique (14), which has been reported to have a high incidence of complications after second-stage foreskin flap repair. The complication rate of two-stage repair in Byars is 68%, with fistula, diverticulum, and urethral stricture being the most common (15). McNamara et al. (16) reported that after a two-stage repair, the complication rate was 53%, with fistula (29.1%) being the most common, and the reoperation rate was 49%. Snodgrass et al. (17) suggested that the Byars flap retained scars in the middle line and had poorer corporal adherence. Their study used the Byars operation in 9 patients and had a 100% complication rate, including 2 fistulas, 5 diverticula, 1 stricture, and 2 cases of glans dehiscence. It was concluded that the diverticulum resulted from the relatively fixed resistance of the glans and/or turbulent flow from the poor fixation of the flap to the corpora, causing the preputial skin to stretch. Therefore, many pediatric surgeons no longer recommend this technique (18). The second technique is staged partial urethroplasty in which preputial flaps are used to create a part of the urethra. Chen et al. (19) performed staged transverse preputial island flap (TPIF) and achieved good results. The reoperation, fistula, and diverticulum rates were 7.1%, 4.8%, and 2.4%, respectively, in the staged TPIF group. In addition, Pfistermüller et al. (20) reported that the modified Ulaanbaatar procedure was used for tubularization of the glans with a TPIF in the first-stage procedure in cases of severe hypospadias. Urethral diverticula occurred in 12% of the patients, requiring reoperation. The success rate of the TPIF or modified Ulaanbaatar procedure is higher than that of the traditional Duckett procedure because this technique creates a controlled fistula in the first stage, which may allow the reconstructed urethra during the first-staged surgery to heal and become adherent to the corpus cavernosum. In the first stage, Byars’ flaps bridge the defect from the original hypospadiac orifice to the neourethra. However, if the flaps do not adhere firmly to the cavernous body, a diverticulum may occur later. This staged technique creates an island flap of preputial skin that is used for tubularization, and therefore the vascular pedicle may cause a penile distortion. The third approach is the Bracka technique, whereby the glans is split, and a ventral free graft (inner preputial or buccal mucosa) is used to cover the defect from the original orifice to the tip of the glans during the first stage. The inner prepuce is excised from the dartos and quilted to the underlying corpora using interrupted 6-0 polyglactin sutures spaced approximately 5 mm apart. These procedures can achieve good graft healing and good corporal adherence. Pfistermüller et al. (20) reported a significant reduction in the incidence of complication rates with an overall reoperation rate of 6.3%, fistula rate of 3.4%, meatal stenosis rate of 1.4%, and partial glans dehiscence rate of 1.4%. However, Joshi et al. (21) reported 100% graft absorption without revision after the first staged surgery. The postoperative fistula rate was 10%, and no urethral stricture, glans dehiscence, or residual chordee was observed during follow-up. The cosmetic results were satisfactory, and the urinary stream was good in all patients. In our study, the success rate of the Bracka group was approximately the same as that reported in previous studies. We also compared the characteristics and effects of the Bracka and Duckett techniques.
There were significant differences in complications between the two groups, with a rate of 13.1% total complications in the staged group and 35.4% in the single-stage group. The major benefit of the Bracka technique was that the new urethra was well fixed to the corpus cavernosum and not prolapsed, as in cases repaired using the Duckett method, which was the main factor that prevented diverticular formation. The diverticular rates in the Bracka and Duckett groups were 0% and 2.1%, respectively. The second stage involved suturing of the reconstructed urethral plate into the neourethra with duplex repair. In the Duckett technique, circular anastomosis between the new urethra and the original urethral orifice is prone to strictures. However, due to semicircular anastomosis, the Duplay procedure significantly reduced the rate of stricture formation. The stricture rates in the Bracka and Duckett groups were 4.3% and 8.3%, respectively. By splitting the glans and filling the graft, a larger and slit-like meatus was achieved. Even patients with small glans can undergo this method and achieve an orthotopic urethral opening. Excellent cosmetic results are also anticipated. A small glans can be easily complicated by dehiscence. The dehiscence rates in the Bracka and Duckett groups were 0% and 8.3%, respectively. In the Bracka group, partial scrotum or perineal urethra were formed during the first stage of the operation, and the distal residual urethra was completed in the second stage. As a result, the incidence of urinary fistulas in the Bracka group was lower than that in the Duckett group (8.7% and 16.7%, respectively). Therefore, two-stage Bracka repair was better than Duckett repair in reducing postoperative complications. Although the graft had no vascular pedicles, we did not observe any graft failure after the first stage of surgery. If partial graft contracture occurred, the second-stage operation could be delayed by 1 year. During the waiting period, a warm water bath and betamethasone ointment were used externally. TIP or regrafting could be used before the second-stage tubularization if scars persist.
Risk factor analysis revealed that urethral defects after correction of the chordee and urethral fistulas were significantly correlated. According to Poiseuille’s law, the pressure difference produced in a tube is proportional to its length and inversely proportional to its radius. Braga et al. (22) showed that the new, long urethra was more prone to urethral fistulas. Theoretically, the length of the urethral defect is approximately equal to the length of the new urethra that requires external coverage. The greater the number of defects, the more outer covering tissue is required. When local tissue cannot provide adequate coverage, the probability of a fistula is significantly increased (23,24). Moreover, the length of the urethral defect is equivalent to the length of the urethra without the support of the urethral plate. Therefore, the longer the new urethra, the higher the probability of a fistula occurring. In order to reduce complications such as urethral fistulas, a well vascularized tissue will be needed to cover the neourethra. At present, the dartos fascia, scrotal fascia, divergent spongiosum, and tunica vaginalis flap are commonly used as covering layers (24,25). In our study, after the neourethra was formed, a protective dartos fascia flap or tunica vaginalis flap was placed over the entire suture line as a waterproof layer.
The retrospective nature of the study was one of its major limitations and led to a less rigorous design than would have been seen in a prospective comparative study. Incomplete information on voiding stream analysis, sexual function, quality of life, and penile cosmetic appearance also limited assessment of the final results. However, given the ongoing debate regarding the best way to treat proximal hypospadias with severe chordee, particularly whether single-stage or two-stage surgery is the preferred option, our results provided information to help surgeons make suitable treatment decisions. Our future research will focus on a prospective randomized controlled trial using different urethroplasty procedures for the treatment of proximal hypospadias with severe chordee.
Conclusions
The Bracka two-stage repair had some advantages: (I) it could be applied in almost all types of proximal hypospadias with good cosmetic results, and particularly in cases of perineal hypospadias, the Bracka two-stage repair should be selected; (II) the Bracka two-stage repair had low complication rates, and the implementation of the Bracka procedure is straightforward if the surgeon has basic hypospadias repair skills, and it had a significantly higher success rate than that of Duckett repair. If complications occur, a third surgery is required. Although either the Bracka two-stage repair or the Duckett repair could be used for proximal hypospadias, the Bracka procedure was relatively simple, reliable, and reproducible, and its outcomes were considered superior to those of Duckett repair. In addition, our results showed that the greater the urethral defect after chordee correction, the greater the possibility of a postoperative urethral fistula.
Acknowledgments
We thank all patients treated in our department and all the doctors and nurses in the department for their wonderful clinical care.
Funding: This work was supported by the Tianjin Science and Technology Program (Nos. 20JCYBJC01240 to Yong Guan and 21JCYBJC01310 to Xin Wang) and the Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-040A).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-75/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-75/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-75/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-75/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Tianjin Children’s Hospital (No. 2022-LXKY-001). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg 2006;41:463-72. [Crossref] [PubMed]
- Geng H, Cheng S, Yang X, et al. The Effect of the Duckett procedure on the Outcome and Prognosis of Children with Suburethral Cleft. Contrast Media Mol Imaging 2022;2022:7444104. [Crossref] [PubMed]
- Cousin I, Basmaison C, Cousin E, et al. Complication rates of proximal hypospadias: meta-analyses of four surgical repairs. J Pediatr Urol 2022;18:587-97. [Crossref] [PubMed]
- Xie Q, Liu Y, Zhao X, et al. The effect of staged TIP urethroplasty on proximal hypospadias with severe chordee. Front Surg 2022;9:892048. [Crossref] [PubMed]
- Badawy H, Orabi S, Hanno A, et al. Posterior hypospadias: Evaluation of a paradigm shift from single to staged repair. J Pediatr Urol 2018;14:28.e1-28.e8. [Crossref] [PubMed]
- Ali MM, Anwar AZ. Experience with modified two stage inner preputial flap for repair of proximal hypospadias with chordee: A single institution study with intermediate follow up. J Pediatr Surg 2022;57:1404-8. [Crossref] [PubMed]
- Manasherova D, Kozyrev G, Nikolaev V, et al. Bracka's Method of Proximal Hypospadias Repair: Preputial Skin or Buccal Mucosa? Urology 2020;138:138-43. [Crossref] [PubMed]
- Snodgrass W, Bush N. TIP hypospadias repair: A pediatric urology indicator operation. J Pediatr Urol 2016;12:11-8. [Crossref] [PubMed]
- Babu R, Chandrasekharam VVS. Meta-analysis comparing the outcomes of single stage (foreskin pedicled tube) versus two stage (foreskin free graft & foreskin pedicled flap) repair for proximal hypospadias in the last decade. J Pediatr Urol 2021;17:681-9. [Crossref] [PubMed]
- Rohatgi M. Hypospadias repair--a new modification of Thiersch-Duplay urethroplasty. Indian J Pediatr 1984;51:181-6. [Crossref] [PubMed]
- Snodgrass W, Koyle M, Manzoni G, et al. Tubularized incised plate hypospadias repair for proximal hypospadias. J Urol 1998;159:2129-31. [Crossref] [PubMed]
- Retik AB, Bauer SB, Mandell J, et al. Management of severe hypospadias with a 2-stage repair. J Urol 1994;152:749-51. [Crossref] [PubMed]
- Springer A, Krois W, Horcher E. Trends in hypospadias surgery: results of a worldwide survey. Eur Urol 2011;60:1184-9. [Crossref] [PubMed]
- Byars LT. Surgical repair of hypospadias. Surg Clin North Am 1950;30:1371-8. [Crossref] [PubMed]
- Stanasel I, Le HK, Bilgutay A, et al. Complications following Staged Hypospadias Repair Using Transposed Preputial Skin Flaps. J Urol 2015;194:512-6. [Crossref] [PubMed]
- McNamara ER, Schaeffer AJ, Logvinenko T, et al. Management of Proximal Hypospadias with 2-Stage Repair: 20-Year Experience. J Urol 2015;194:1080-5. [Crossref] [PubMed]
- Snodgrass W, Decter RM, Roth DR, et al. Management of the penile shaft skin in hypospadias repair: alternative to Byars' flaps. J Pediatr Surg 1988;23:181-2. [Crossref] [PubMed]
- Jayanthi VR, Ching CB, DaJusta DG, et al. The modified Ulaanbaatar procedure: Reduced complications and enhanced cosmetic outcome for the most severe cases of hypospadias. J Pediatr Urol 2017;13:353.e1-7. [Crossref] [PubMed]
- Chen C, Yang TQ, Chen JB, et al. The Effect of Staged Transverse Preputial Island Flap Urethroplasty for Proximal Hypospadias with Severe Chordee. J Urol 2016;196:1536-40. [Crossref] [PubMed]
- Pfistermüller KL, Manoharan S, Desai D, et al. Two-stage hypospadias repair with a free graft for severe primary and revision hypospadias: A single surgeon's experience with long-term follow-up. J Pediatr Urol 2017;13:35.e1-7. [Crossref] [PubMed]
- Joshi RS, Bachani MK, Uttarwar AM, et al. The Bracka two-stage repair for severe proximal hypospadias: A single center experience. J Indian Assoc Pediatr Surg 2015;20:72-6. [Crossref] [PubMed]
- Braga LH, Pippi Salle JL, Lorenzo AJ, et al. Comparative analysis of tubularized incised plate versus onlay island flap urethroplasty for penoscrotal hypospadias. J Urol 2007;178:1451-6; discussion 1456-7. [Crossref] [PubMed]
- Fahmy O, Khairul-Asri MG, Schwentner C, et al. Algorithm for Optimal Urethral Coverage in Hypospadias and Fistula Repair: A Systematic Review. Eur Urol 2016;70:293-8. [Crossref] [PubMed]
- Yang F, Ruan J, Zhao Y, et al. Individual treatment strategy for single urethrocutaneous fistula after hypospadias repair: a retrospective cohort study. Transl Androl Urol 2022;11:1345-53. [Crossref] [PubMed]
- Yiğit D, Avlan D. Dorsal versus ventral dartos flap to prevent fistula formation in tubularized incised plate urethroplasty for hypospadias. Urol J 2022;19:315-9. [PubMed]
(English Language Editor: A. Muijlwijk)


