Comparison of Doppler echocardiographic and tissue Doppler velocity data in beta-thalassaemia major with high and normal NT-proBNP levels of children in the south-east region of Turkey
Introduction
The β-thalassemia major (β-TM) is a hereditary chronic hemolytic anemia that typically requires lifelong regular transfusion therapy to keep hemoglobin levels close to normal and allow adequate tissue oxygenation. The chronic administration of large amount of blood together with extravascular hemolysis and increased iron absorption in the intestinal system leads to iron deposition into the heart, liver, lung etc. in patients with β-TM (1,2). Iron induced cardiomyopathy is still major cause of morbidity and mortality in transfusion dependent β-TM and it can be reversible only if early intensive chelation therapy has been initiated. Also, the iron induced cardiomyopathy is silent in young patients with β-TM and conventional echocardiography is not sensitive for early diagnosis of the preclinical stage of cardiac involvement (3). Currently, cardiac magnetic resonance T2* has been proven as the most sensitive index to asses cardiac iron, and it has become a widely used technique in developed countries (4,5). Together with that, N-terminal pro-brain natriuretic peptide (NT-proBNP) has been reported as an early biomarker of iron- induced cardiomyopathy in β-TM in several studies (6,7). In this study, we aimed to assess the Doppler echocardiographic and tissue Doppler velocity data of β-TM patients with high and normal NT-proBNP levels who have normal systolic function.
Methods
Study population
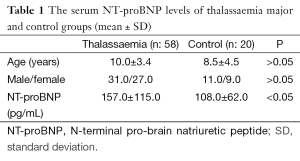
Fifty-eight β-TM patients (4-17 years of age; 31 males, 27 females) with normal ventricular systolic function as assessed by conventional echocardiography and 20 healthy subjects (3-17 years of age; 11 males/9 females) were included into the study. The β-TM patients were on regular blood transfusion program in every 3-4 weeks for more than one year (range: 1-16 years; mean: 8.9±3.5 years). Before they were on oral chelation therapy with deferasirox 20-40 mg/kg/day over one and a half year, they were poorly chelated. The mean serum ferritin value was calculated from measurements of last 3 months period. All β-TM patients underwent a through clinical and echocardiographic examination and blood samples were collected for whole blood counts, biochemical parameters, NT-proBNP on pre-transfusion day. NT-proBNP levels of β-TM and control group were measured with Immulite 2000, DPC (Los Angeles, CA, USA) (a solid phase, two-site chemiluminescence immunometric assay). The upper limit of the reference range for NT-proBNP was 125 pg/dL. According to NT-proBNP levels, β-TM patients are divided in two groups: Group I: the patients with high NT-proBNP levels; Group II: the patients with normal NT-proBNP levels. The study protocol was approved by the Institutional Ethics Committees (IECs) and written informed consent was obtained from all patients.
Echocardiographic study
All echocardiographic measurements were obtained by using General Electric (GE) Vivid S5 ultrasound machine equipped with a 3 MHz transducer. The heart was scanned from all standard views and five cardiac cycle loops from each view were digitally stored on VHS videotape and analyzed at study completion. Standard trans-thoracic echocardiogram (TTE) involved M-mode, two-dimensional Doppler flow assessments, and tissue Doppler indices (TDI) measurements. Measurements of cavity dimensions were carried out according to the American Society of Echocardiography (ASE) guidelines. Left ventricular (LV) mass was calculated by the method of Devereux and Reichek and indexed to body surface area (BSA). LV diastolic inflow velocities were obtained from apical four-chamber view by placing the sample volume at the valve tips level. Peak early mitral inflow velocity (E) (cm/s), peak late atrial mitral inflow velocity (A) (cm/s), TDI velocities of mitral-tricuspid annular, septal motion were recorded by using optimal filters and scale. Care was taken for the ultrasound beam to be parallel to the septal annular border. Systolic (S′), early diastolic (E′), and late diastolic velocities (A′) were measured. The ratio of early diastolic mitral and tricuspid inflow velocity to early diastolic TDI mitral and tricuspid annular velocity (E/E′) was calculated. Ejection fraction (EF) was calculated by modified Simpson’s rule.
Statistical analysis
All statistical procedures were performed using Statistical Package for Social Sciences (SPSS) Windows Version 17.0 (SPSS Inc., Chicago, IL, USA). Data was summarized by mean ± standard deviation (SD) unless otherwise stated. Statistically significant differences between groups of continuous variables were determined by using students’ t-test. The degree of association between echocardiographic findings with NT-proBNP levels were determined using Pearson correlation test. P value <0.05 was considered statistically significant.
Results
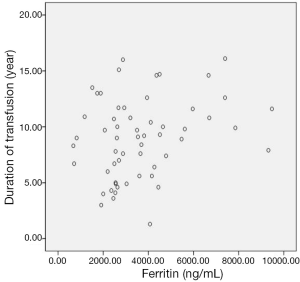
Age, gender, and NT-proBNP levels of β-TM patients and healthy subjects were shown in Table 1. There were not any differences between control group and patients with β-TM in terms of age and gender. Serum NT-proBNP levels were ranged 24-494 pg/dL (mean: 157±115 pg/dL) in β-TM patients and 28-213 pg/dL (mean: 108±62 pg/dL) in control group. This difference was statistically significant (P<0.05) (Table 1). All of β-TM patients’ conventional echocardiography evaluation revealed no cardiac dysfunction and their mean EF were estimated to be 71.8%±7.3%. Their serum ferritin levels were ranged between 676-9,476 ng/mL (mean: 3,716±2,003 ng/mL) and it was positively correlated with transfusion duration (r: 0.267) (Figure 1), but was not correlated with NT-proBNP.
Full table
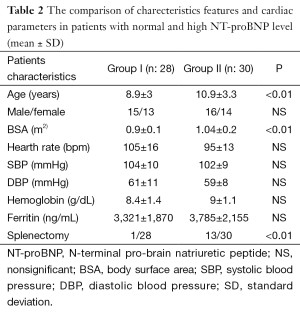
The characteristic features of the patients in Group I and Group II are summarized in Table 2. The splenectomised patients were 1/28 (3.5%) in group I and 13/30 (43%) in group II. The mean NT-proBNP levels were 274±108 pg/mL in Group I and 83.5±26 pg/mL in Group II. The mean age and BSA were significantly low in Group I compared to Group II (P<0.01). The mean heart rate, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were elevated, but not significantly in group I. Also, the mean ferritin and hemoglobin were low in this group compared to group II, but statistically not significant. No correlation was found between NT-proBNP with these parameters.
Full table
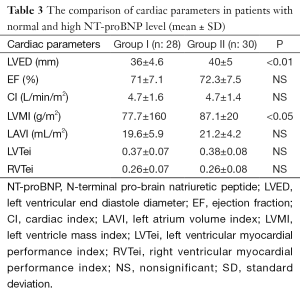
EF, cardiac index (CI), left atrium volume index (LAVI), left ventricular myocardial performance index (LVTei), and right ventricular myocardial performance index (RVTei) were not statistically different in both groups. The mean left ventricular end diastole (LVED) diameters and left ventricular mass index (LVMI) values were found significantly low in group I compared to other group respectively (P<0.001, P<0.05) (Table 3).
Full table
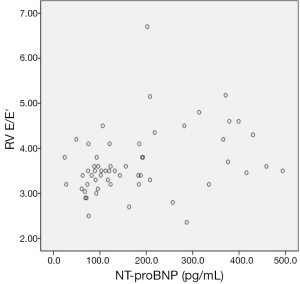
The mean Doppler echocardiographic and tissue Doppler velocity data are shown in Table 4. Only, the mean right ventricular early diastolic tricuspid inflow velocity/early diastolic TDI tricuspid annular velocity (RV E/E′) were found increased in group I and difference was statistically significant (P<0.05). In addition, NT-pro BNP was found correlated with only RV E/E′ (r: 0.320) (Figure 2). The other Doppler echocardiographic and tissue Doppler velocity data was not different in both groups (Table 4).
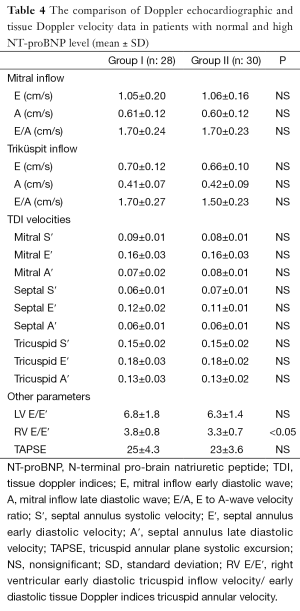
Full table
Discussion
Iron cardiomyopathy is still the leading cause of death in patients with thalassemia major (8). First in 1964, Engle and et al. observed that patients who died of congestive heart failure had a wide range of iron transfused (3-104 gr) and age (6 to 31 years). Later, its etiology was documented apart from the direct toxic effect of iron deposited in the myocardium, immune mediated myocardial dysfunction, repetitive antigenic stimulus due to multi-blood transfusions and chelation therapy (9-13).
Brain natriuretic peptide (BNP) and amino-terminal fragment of proBNP (NT-proBNP) are mainly released in response to increased cardiac volume and pressure overload. They decrease blood pressure by reducing preload and increase venous capacitance, which helps to facilitate natriuresis that reduces the overall extracellular fluid volume. NT-proBNP and BNP have emerged as sensitive biomarkers for the diagnosis, prognosis, and treatment of heart failure. Also, NT-proBNP seems to be a reliable index for detecting isolated diastolic dysfunction. It has been reported that as an early biomarker of LV diastolic dysfunction, compared with the conventional echo-Doppler indexes in β-TM in lots of studies (7,14-18).
The left-sided heart failure is seen clinically more common than right-sided heart failure in children with β-TM, but right ventricular dysfunction may develop earlier in asymptomatic β-TM patients (9,12,13,17). In present study, NT-proBNP level was found positively correlated with RV E/E′ in β-TM patients (r: 320) (P<0.05). Also, RV E/E′ in Group I was higher than Group II (P<0.05). These findings may be related with right ventricular diastolic dysfunction in asymptomatic patients with β-TM in our study. On the other hand, the increased wall thickness and mass are characteristics of cardiomyopathy in thalassemia major at advanced stage (19). In our study, LVED and LVMI values were not found increased in group with high NT-proBNP levels comparing to group’s with normal NT-proBNP levels. It was thought low values might be related with younger population in group I compared to II.
High output state related with chronic anemia and frequent blood transfusion might be caused to cardiac stretching and overload as a responsible factor for the secretion of NT-proBNP (10,11). In present study, hemoglobine level was low and hearth rate was high in group I, but not statistically significant. The increased RVE/E′, low hemoglobin and increased heart rate may reflect increased myocardial workload and increased need for transfusion. Each group transfusion index data was not available, but splenectomised patients in group II was higher than group II, approximately 43% (13/30). Splenectomy might be decreased the need for transfusion in group II, because its mean hemoglobin level was not as low as compared to group I.
Serum ferritin is inexpensive and accessible worldwide. It reflects 1% of the total iron storage pool and is the most common laboratory parameter that is used to estimate of body iron stores but it may be complicated by a variety of conditions such as infection and heart failure. The recent studies showed that ferritin level was not correlated well with cardiac iron burden (4,8,20). In our study, we could not be able to measure cardiac iron store due to technical problems. In contrast to Kremastinos et al.’s (21,22) results, no correlation was determined between ferritin and NT-proBNP in present study. Like it was stated in a study (1), the reason for the lack of this correlation may be that the serum ferritin levels of our population were based on last 3 months, while those of the previous studies were derived as average values from the 3 months intervals over the last 5- and 4-year-period, respectively.
Our study has some limitations. First, we could not measure iron overload with cardiovascular T2-star (T2*) magnetic resonance which is known as a gold standard for early diagnosis of myocardial iron overload. Second, we could not perform the Doppler echocardiography and tissue Doppler echocardiography velocities of healthy subjects to obtain more accurate cardiac parameters according to age.
Conclusions
According to our results, elevated NT-proBNP level was correlated with RVE/E′, but it was not associated with ferritin level. The serum NT-proBNP level may be increased as a response to increased myocardial workload and decreased hemoglobin level in patients who have an increased need for transfusion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Delaporta P, Kattamis A, Apostolakou F, et al. Correlation of NT-proBNP levels and cardiac iron concentration in patients with transfusion-dependent thalassemia major. Blood Cells Mol Dis 2013;50:20-4. [PubMed]
- Fourcade L, Paule P, Mioulet D, et al. Thalassemia cardiomyopathy. Med Trop (Mars) 2007;67:617-9. [PubMed]
- Koonrungsesomboon N, Chattipakorn SC, Fucharoen S, et al. Early detection of cardiac involvement in thalassemia: From bench to bedside perspective. World J Cardiol 2013;5:270-9. [PubMed]
- Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001;22:2171-9. [PubMed]
- Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009;120:1961-8. [PubMed]
- Aessopos A, Farmakis D, Polonifi A, et al. Plasma B-type natriuretic peptide concentration in beta-thalassaemia patients. Eur J Heart Fail 2007;9:537-41. [PubMed]
- Balkan C, Tuluce SY, Basol G, et al. Relation between NT-proBNP levels, iron overload, and early stage of myocardial dysfunction in β-thalassemia major patients. Echocardiography 2012;29:318-25. [PubMed]
- Wood JC, Enriquez C, Ghugre N, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann N Y Acad Sci 2005;1054:386-95. [PubMed]
- Cohen AR, Galanello R, Pennell DJ, et al. Thalassemia. Hematology Am Soc Hematol Educ Program 2004:14-34.
- Engle MA, Erlandson M, Smith CH. Late Cardiac Complications of Chronic, Severe, Refractory Anemia with Hemochromatosis. Circulation 1964;30:698-705. [PubMed]
- Kremastinos DT, Tsetsos GA, Tsiapras DP, et al. Heart failure in beta thalassemia: a 5-year follow-up study. Am J Med 2001;111:349-54. [PubMed]
- Spirito P, Lupi G, Melevendi C, et al. Restrictive diastolic abnormalities identified by Doppler echocardiography in patients with thalassemia major. Circulation 1990;82:88-94. [PubMed]
- Vogel M, Anderson LJ, Holden S, et al. Tissue Doppler echocardiography in patients with thalassaemia detects early myocardial dysfunction related to myocardial iron overload. Eur Heart J 2003;24:113-9. [PubMed]
- Aessopos A, Deftereos S, Tsironi M, et al. Predictive echo-Doppler indices of left ventricular impairment in B-thalassemic patients. Ann Hematol 2007;86:429-34. [PubMed]
- Aessopos A, Giakoumis A, Fragodimitri C, et al. Correlation of echocardiography parameters with cardiac magnetic resonance imaging in transfusion-dependent thalassaemia major. Eur J Haematol 2007;78:58-65. [PubMed]
- Kremastinos DT. Heart failure in beta-thalassemia. Congest Heart Fail 2001;7:312-4. [PubMed]
- Kremastinos DT, Tsiapras DP, Kostopoulou AG, et al. NT-proBNP levels and diastolic dysfunction in beta-thalassaemia major patients. Eur J Heart Fail 2007;9:531-6. [PubMed]
- Lekawanvijit S, Chattipakorn N. Iron overload thalassemic cardiomyopathy: iron status assessment and mechanisms of mechanical and electrical disturbance due to iron toxicity. Can J Cardiol 2009;25:213-8. [PubMed]
- Henry WL, Nienhuis AW, Wiener M, et al. Echocardiographic abnormalities in patients with transfusion-dependent anemia and secondary myocardial iron deposition. Am J Med 1978;64:547-55. [PubMed]
- Letsky EA, Miller F, Worwood M, et al. Serum ferritin in children with thalassaemia regularly transfused. J Clin Pathol 1974;27:652-5. [PubMed]
- Kremastinos DT. Beta-thalassemia heart disease: is it time for its recognition as a distinct cardiomyopathy? Hellenic J Cardiol 2008;49:451-2. [PubMed]
- Kremastinos DT, Farmakis D, Aessopos A, et al. Beta-thalassemia cardiomyopathy: history, present considerations, and future perspectives. Circ Heart Fail 2010;3:451-8. [PubMed]